
G1 Phase: The First Step of Interphase
The G1 phase, or Gap 1 phase, is the first stage of interphase in the cell cycle. It is a period of cell growth, metabolic activity, and preparation for DNA synthesis. This phase is crucial for ensuring that the cell is adequately prepared for the subsequent phases of the cell cycle, particularly the DNA replication that occurs in the S phase.
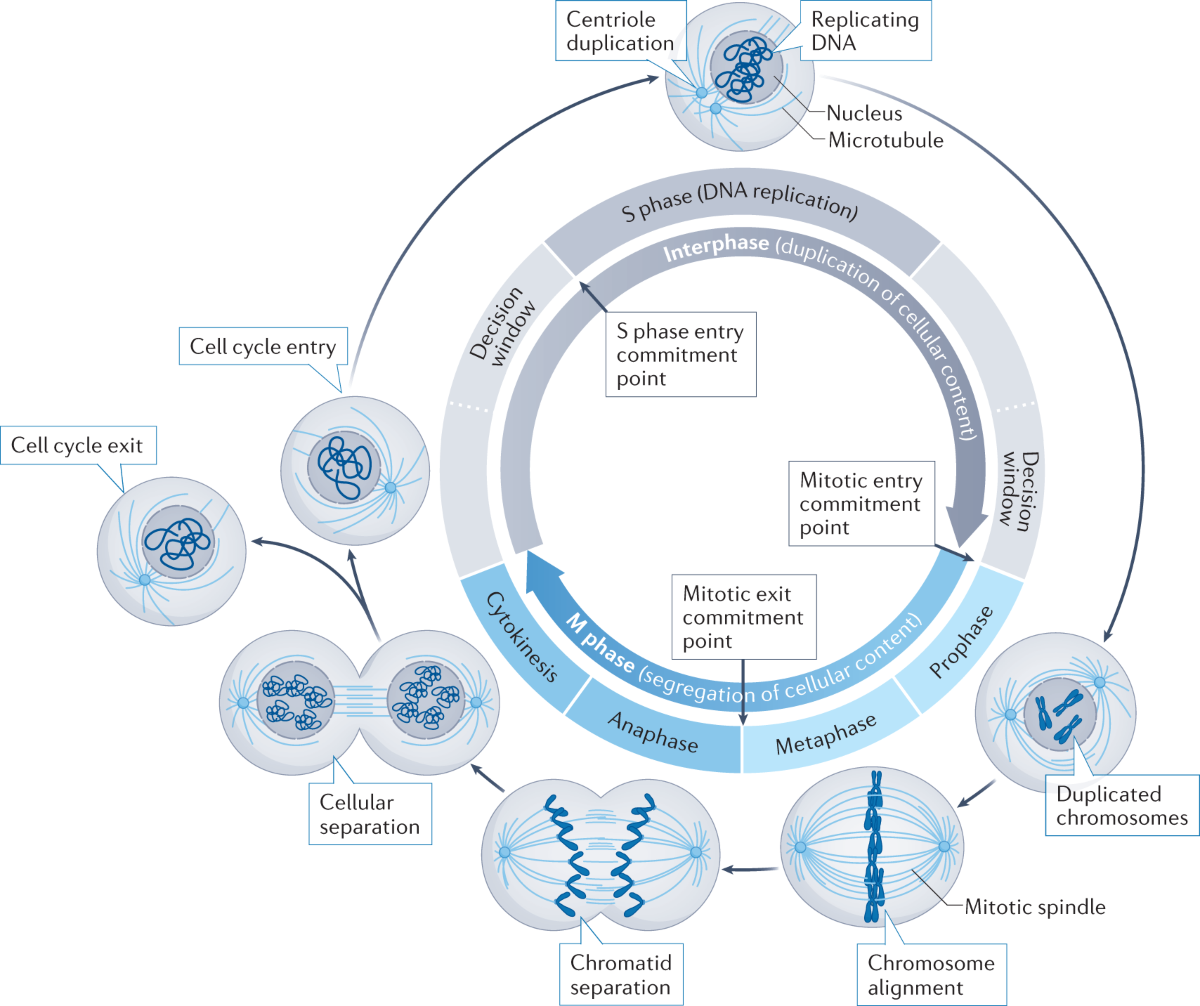
Characteristics of the G1 Phase
Cell Growth and Biosynthesis
During the G1 phase, the cell undergoes significant growth. This growth is necessary to ensure that the cell reaches an adequate size to divide and produce two viable daughter cells. Key processes during this phase include:
- Increase in Cell Size: The cell increases its volume by synthesizing cytoplasm and expanding the plasma membrane.
- Organelle Duplication: Cells prepare for DNA replication and division by duplicating organelles. Mitochondria, the endoplasmic reticulum, and Golgi apparatus expand and replicate to ensure each daughter cell receives the necessary components. This process is regulated by growth factor signaling and cellular checkpoints, ensuring that the cell's organelle content is sufficient for subsequent phases, particularly for maintaining metabolic and structural functions in both daughter cells.
- Protein and Enzyme Synthesis: The cell synthesizes a variety of proteins and enzymes required for DNA replication and other cellular functions. This includes enzymes involved in metabolism, DNA repair, and replication.
Metabolic Activity
The G1 phase is marked by heightened metabolic activity to support cell growth and prepare for DNA synthesis. This involves:
- Energy Production: Increased activity in metabolic pathways such as glycolysis, the citric acid cycle, and oxidative phosphorylation to generate ATP.
- Macromolecule Synthesis: Cells actively synthesize macromolecules essential for growth and preparation for DNA replication. This includes the production of proteins, lipids, and carbohydrates. Ribosomes and endoplasmic reticulum are highly active, generating structural proteins and enzymes. Additionally, nucleotides necessary for DNA synthesis in the upcoming S phase are synthesized. This macromolecule synthesis ensures the cell has the resources needed for successful progression through the cell cycle.
Regulatory Mechanisms
Cell Cycle Checkpoints
The G1 phase contains a critical checkpoint, known as the G1 checkpoint or restriction point, that determines whether the cell is ready to proceed to the S phase. This checkpoint assesses several factors:
- Cell Size: The cell must be large enough to proceed to DNA replication.
- Nutrient Availability: Adequate nutrients and growth factors must be present to support cell growth and division.
- DNA Integrity: The cell's DNA must be undamaged. If there is DNA damage, the cell cycle can be halted to allow for repair.
Molecular Regulators
The progression through the G1 phase and passage through the G1 checkpoint are tightly controlled by a network of molecular regulators:
- Cyclins and Cyclin-Dependent Kinases (CDKs): Cyclin D binds to CDK4/6, forming active complexes that phosphorylate the retinoblastoma protein (Rb). This releases E2F transcription factors, initiating the transcription of genes required for S phase entry. Cyclin E-CDK2 further phosphorylates Rb, ensuring complete transition to the S phase, thereby tightly regulating cell cycle progression.
- Retinoblastoma Protein (Rb): The Rb protein is a key regulator that inhibits cell cycle progression by binding to and inhibiting E2F transcription factors. Phosphorylation of Rb by cyclin-CDK complexes releases E2F, allowing the transcription of genes necessary for S phase entry.
- Tumor Suppressors: Proteins like p53 monitor DNA integrity and can halt the cell cycle if damage is detected. Activation of p53 can lead to the expression of p21, a CDK inhibitor, which prevents the phosphorylation of Rb and blocks cell cycle progression.
Cellular Responses to Environmental Signals
Cells in the G1 phase are highly responsive to external signals, such as growth factors and nutrients. These signals influence whether the cell will continue to divide or enter a quiescent state (G0 phase):
- Growth Factors: Binding of growth factors to cell surface receptors activates intracellular signaling pathways (e.g., the MAPK/ERK pathway) that promote cell cycle progression.
- Nutrient Sensing: The availability of essential nutrients, such as glucose and amino acids, is monitored by cellular pathways (e.g., mTOR pathway) that integrate environmental signals and coordinate cellular growth with nutrient availability.
G0 Phase: Quiescence
The G0 phase, often referred to as the quiescent stage, is a period in the cell cycle where cells exit the active cycle and enter a state of dormancy. Cells in G0 are not actively preparing to divide; instead, they perform their regular functions. This phase can be temporary or permanent. Temporary G0 allows cells to re-enter the cycle under appropriate conditions, while permanent G0, or terminal differentiation, is seen in specialized cells like neurons and muscle cells, which do not divide. The transition into and out of G0 is regulated by various signals, including nutrient availability, growth factors, and cellular stress, ensuring proper tissue function and regeneration.

Importance of G1 Phase Regulation
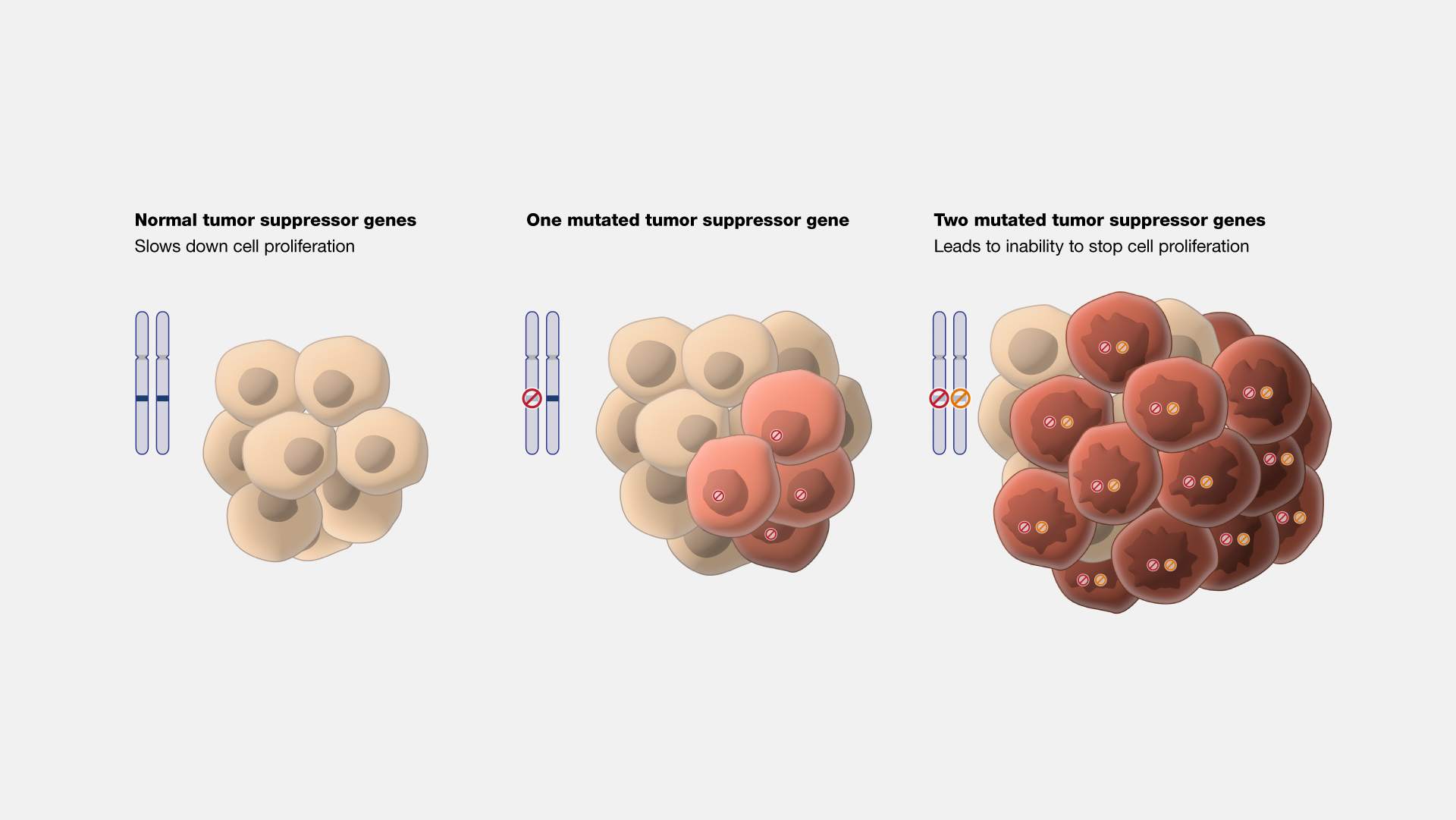
Proper regulation of the G1 phase is essential for maintaining cellular and organismal health. Dysregulation can lead to uncontrolled cell proliferation and cancer. For instance:
- Oncogenes: Mutations that result in the overactivation of cyclins, CDKs, or growth factor signaling can drive uncontrolled cell division.
- Tumor Suppressors: Loss of function in tumor suppressors like p53 or Rb can remove critical checks on cell cycle progression, leading to unchecked growth and cancer development.
G1 Phase FAQ
The transition from quiescence (G0 phase) to the G1 phase is tightly regulated by various signaling pathways and transcriptional regulators. These include growth factor signaling pathways (e.g., PI3K-Akt, MAPK/ERK), cyclin-dependent kinases (CDKs), and transcription factors (e.g., Myc, E2F). Understanding how these regulatory networks integrate external signals and internal cues to control cell cycle entry is essential for deciphering cell fate decisions, such as proliferation, differentiation, or senescence.
Metabolic reprogramming and nutrient availability play crucial roles in regulating cell cycle entry and progression through the G1 phase. Growth factor signaling pathways, such as the PI3K-Akt-mTOR pathway, integrate nutrient and energy status to promote cell growth and proliferation. Nutrient sensing mechanisms, including AMP-activated protein kinase (AMPK) and mTOR complex 1 (mTORC1), regulate the expression and activity of cell cycle regulators like cyclins and CDKs. Understanding how metabolic cues are integrated with cell cycle signaling pathways during the G1 phase provides insights into the coordination of cellular growth and proliferation in physiological and pathological conditions.
The G1 phase progression and the G1/S transition are primarily regulated by the Cyclin D-CDK4/6 and Cyclin E-CDK2 complexes. These kinases phosphorylate the retinoblastoma protein (Rb), releasing E2F transcription factors that activate genes necessary for DNA replication. The PI3K/AKT pathway and growth factor signaling also play significant roles by promoting Cyclin D expression and CDK activation.
The G1 checkpoint, also known as the restriction point, ensures DNA integrity by halting cell cycle progression in response to DNA damage or incomplete replication signals. The tumor suppressor protein p53 plays a crucial role in this process. Upon DNA damage, p53 is stabilized and activates the transcription of p21, an inhibitor of Cyclin-CDK complexes, preventing the G1/S transition until the damage is repaired.
The retinoblastoma protein (Rb) acts as a key regulator of the G1 phase by controlling the activity of E2F transcription factors. In its hypophosphorylated state, Rb binds to E2F, inhibiting its activity and preventing the transcription of genes required for S phase entry. Phosphorylation of Rb by Cyclin D-CDK4/6 and Cyclin E-CDK2 during the G1 phase leads to the release of E2F, allowing the cell cycle to progress.
Environmental signals such as nutrient availability, growth factors, and cellular stress significantly influence the G1 phase length and progression. Growth factor signaling through receptors like EGFR activates downstream pathways (e.g., PI3K/AKT, MAPK) that promote Cyclin D expression and CDK activation. Intracellular signals, including the energy status of the cell (AMP/ATP ratio) sensed by AMPK, can also modulate G1 progression by influencing metabolic pathways and cell cycle regulators.
Dysregulation of the G1 phase is commonly linked to cancer due to mutations in genes encoding Cyclins, CDKs, Rb, and p53, leading to uncontrolled cell proliferation. Therapeutic strategies targeting the G1 phase include CDK4/6 inhibitors (e.g., palbociclib, ribociclib), which specifically inhibit the Cyclin D-CDK4/6 complex, thereby restoring control over the G1/S transition and slowing down the proliferation of cancer cells. These therapies are particularly effective in cancers with intact Rb protein.
Conclusion
The G1 phase is a pivotal stage in the cell cycle, characterized by cell growth, metabolic activity, and preparation for DNA replication. It is tightly regulated by checkpoints, cyclins, CDKs, and tumor suppressors to ensure that the cell is ready for the subsequent phases of the cell cycle. Proper regulation of the G1 phase is crucial for normal cellular function and the prevention of diseases such as cancer. Understanding the intricacies of the G1 phase provides insights into cellular behavior and the basis for targeted therapeutic interventions.
You can read this book on G1 Phase https://link.springer.com/article/10.1007/s00018-007-7271-z, which can expand your knowledage.
You can also check out this article on G1-phase where you can find some more information





