Anaphase: The Separation of Chromatids
Anaphase is a crucial phase of mitosis and meiosis during which sister chromatids or homologous chromosomes are pulled apart to opposite poles of the cell. This phase follows metaphase and precedes telophase. Anaphase ensures that each daughter cell receives an identical set of chromosomes, maintaining genetic stability across cell divisions.

Key Events in Anaphase
Anaphase is a critical phase of mitosis during which replicated chromosomes are separated into two identical sets, eventually forming the basis of two new nuclei in the daughter cells. Here are the key events in anaphase, along with their descriptions:
-
Initiation of Anaphase
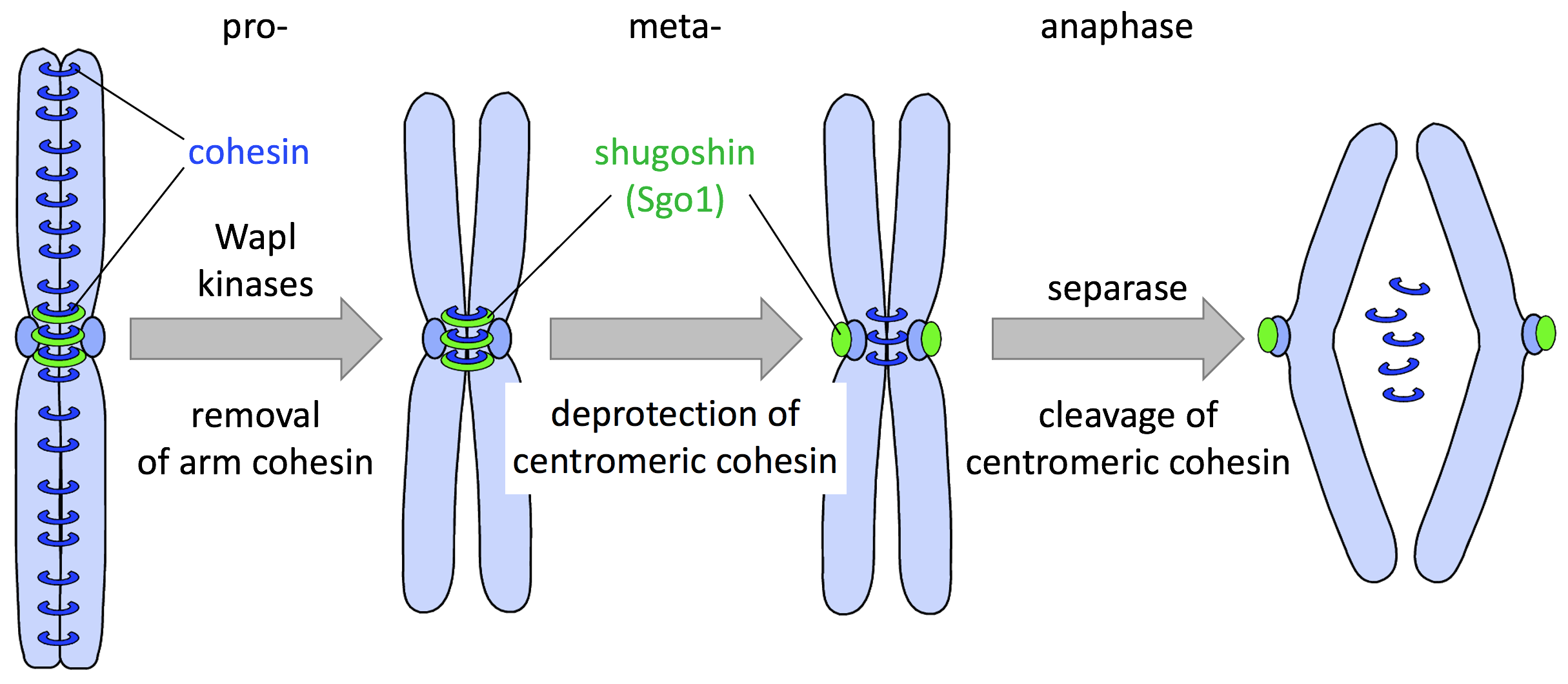
- Anaphase Promoting Complex/Cyclosome (APC/C): The transition from metaphase to anaphase is triggered by the activation of the APC/C. APC/C is a ubiquitin ligase that targets securin and cyclin B for degradation. Securin inhibits separase, an enzyme responsible for cleaving cohesin proteins that hold sister chromatids together. Once securin is degraded, separase becomes active.
- Cohesin Cleavage: Activated separase cleaves the cohesin complexes, allowing sister chromatids to separate and move toward opposite poles.
-
Chromatid Separation
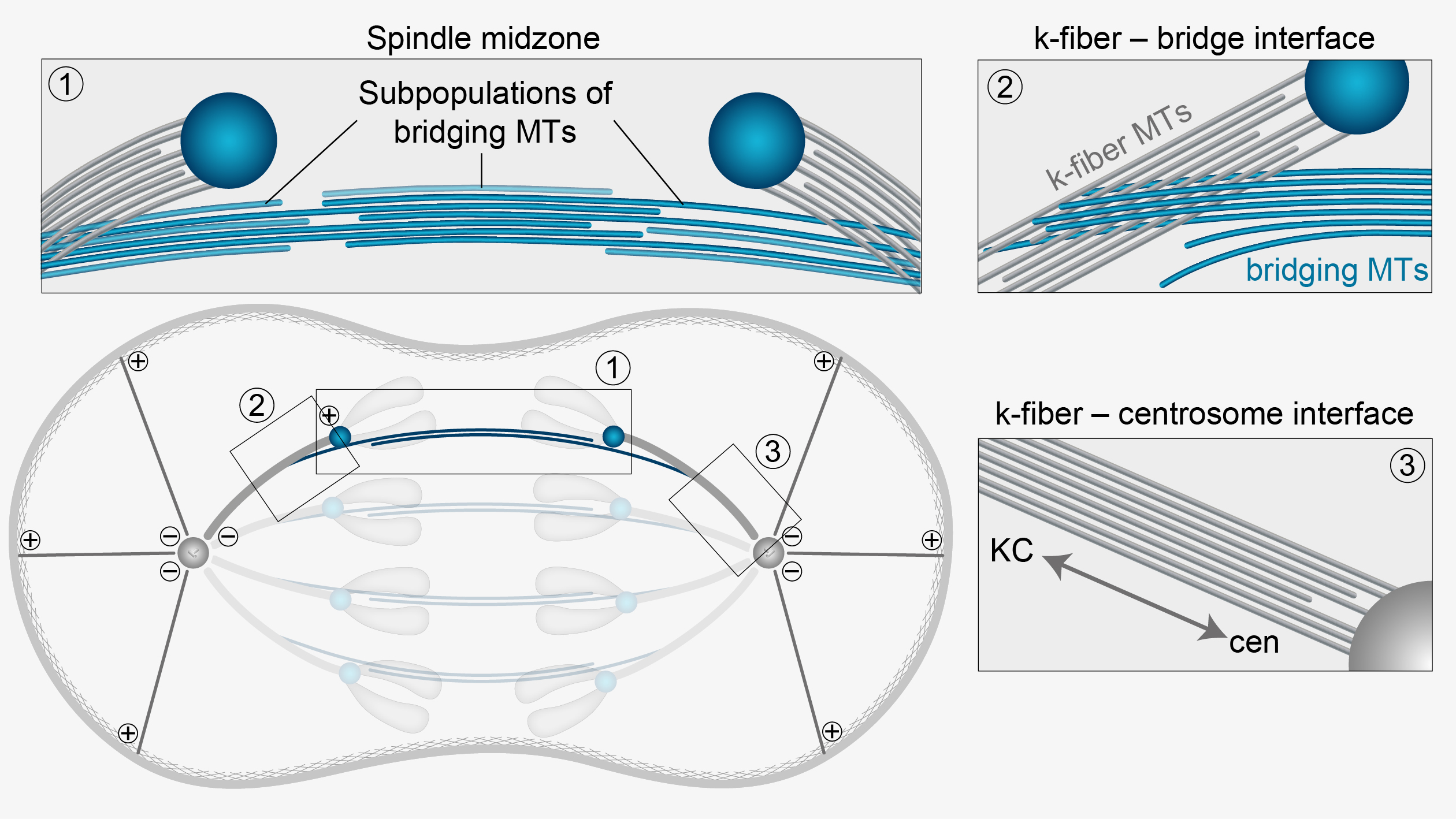
- Anaphase A: This sub-phase involves the shortening of kinetochore microtubules, pulling sister chromatids toward the spindle poles. Motor proteins like dynein and kinesin play crucial roles in this process, moving chromatids along the microtubules.
- Anaphase B: This sub-phase involves the elongation of the cell as polar microtubules slide past each other, driven by motor proteins like kinesin-5. This elongation helps to separate the spindle poles further apart, ensuring that the chromatids are well-separated before cell division completes.

-
Chromosome Movement - Poleward Movement: During anaphase, sister chromatids move rapidly toward the spindle poles. This movement is facilitated by the depolymerization of kinetochore microtubules and the action of motor proteins. - Spindle Elongation: Concurrently, the spindle apparatus elongates, increasing the distance between the poles and contributing to the separation of chromatids.
-
Formation of Daughter Chromosomes:
- Each separated chromatid is now considered an individual chromosome.Once the sister chromatids separate, they are referred to as daughter chromosomes. This ensures that each daughter cell will receive an identical set of chromosomes.
-
Movement Regulation:
- Coordination and regulation of chromosome movement.The cell’s checkpoint mechanisms ensure that all chromosomes are properly attached to the spindle apparatus and that they move accurately. This regulation prevents errors in chromosome distribution, which could lead to aneuploidy.
-
Completion of Chromosome Segregation:
- Chromosomes reach the spindle poles.Once the chromosomes have been pulled to the opposite ends of the cell, they reach the regions near the spindle poles, marking the end of anaphase. The cell is now set for the final stages of mitosis.
Anaphase is followed by telophase, during which the chromosomes begin to decondense, and the nuclear envelope reforms around each set of chromosomes, completing the process of nuclear division.
Molecular Mechanisms Regulating Anaphase
The regulation of anaphase is complex and involves several key molecular mechanisms:
-
Activation of the Anaphase-Promoting Complex/Cyclosome (APC/C)
The APC/C is an E3 ubiquitin ligase that marks specific proteins for degradation by the proteasome. Its activation is crucial for the progression of anaphase.
- Cdc20 and Cdh1: These are co-activators of APC/C. Cdc20 activates APC/C early in anaphase, leading to the degradation of securin and cyclin B, which are inhibitors of anaphase.
- Securin Degradation: Securin inhibits separase, an enzyme that cleaves cohesin. APC/C-mediated ubiquitination and degradation of securin release separase to cleave cohesin and allow sister chromatids to separate.
-
Cohesin Complex Cleavage
Cohesin is a protein complex that holds sister chromatids together. Its cleavage is a pivotal event in anaphase.
- Separase Activation: Activated separase cleaves the kleisin subunit of cohesin (Scc1), thereby releasing the sister chromatids.
-
Spindle Assembly Checkpoint (SAC)
The SAC ensures that all chromosomes are properly attached to the spindle microtubules before anaphase begins. It inhibits APC/C until all kinetochores are correctly attached.
- Mad2, BubR1, and Bub3 Proteins: These proteins inhibit APC/C-Cdc20 by forming the mitotic checkpoint complex (MCC) until all chromosomes are correctly bi-oriented on the mitotic spindle.
- Tension and Kinetochore Attachment: Proper tension and attachment of kinetochores release the inhibition on APC/C-Cdc20, allowing anaphase to proceed.
-
Mitotic Spindle Dynamics
Proper functioning and dynamics of the mitotic spindle are essential for chromosome segregation.
- Kinesin and Dynein Motor Proteins: These proteins move chromosomes along microtubules and are essential for the poleward movement of chromosomes.
- Microtubule Depolymerization: Microtubules depolymerize at the kinetochores, generating forces that pull chromatids apart.
-
Aurora B Kinase and Chromosomal Passenger Complex (CPC)
Aurora B kinase, a part of the CPC, plays a crucial role in correcting improper kinetochore-microtubule attachments and ensuring proper chromosome bi-orientation.
- Error Correction: Aurora B kinase phosphorylates kinetochore proteins, leading to the destabilization of incorrect attachments, which are then corrected for proper chromosome segregation.
-
Cytokinesis Initiation
The successful completion of anaphase is tightly coupled with the initiation of cytokinesis, the process of dividing the cell cytoplasm.
- Central Spindle Formation: The central spindle is formed by overlapping microtubules from opposite poles, and proteins like PRC1 and MKLP1 help organize this structure.
- Cleavage Furrow Formation: Actomyosin contractile ring forms at the cell equator, initiated by signals such as RhoA activation, leading to the cleavage furrow that eventually divides the cell.
-
Mitotic Exit Network (MEN) and NoCut Pathway
The MEN and NoCut pathway ensure proper completion of mitosis and cytokinesis, preventing anaphase progression issues.
- MEN Pathway: In yeast, this pathway regulates the final stages of mitosis, including cytokinesis and mitotic exit.
- NoCut Pathway: Monitors the completion of chromosome segregation before cytokinesis proceeds, ensuring no chromosomal bridges remain.
-
Role of Protein Phosphatases
Phosphatases counteract kinase activities to regulate mitotic progression.
- PP1 and PP2A: These phosphatases dephosphorylate key mitotic substrates, helping to reverse mitotic phosphorylation events and facilitate mitotic exit and cytokinesis.
-
Checkpoints and Feedback Mechanisms
Anaphase regulation includes multiple feedback mechanisms to ensure fidelity.
- Spindle Position Checkpoint: Ensures that the spindle is correctly oriented before cytokinesis.
- Mitotic Exit Checkpoint: Monitors the completion of chromosome segregation and spindle disassembly before allowing exit from mitosis.
These molecular mechanisms work in concert to ensure that anaphase proceeds correctly, leading to the accurate distribution of genetic material between daughter cells. The coordination and regulation of these pathways are crucial for maintaining genomic stability and preventing aneuploidy, which can lead to various diseases, including cancer.
Anaphase in Meiosis
In meiosis, anaphase occurs in two stages:
-
Anaphase I
- Homologous Chromosome Separation: In meiosis I, homologous chromosomes (each consisting of two sister chromatids) are separated and pulled to opposite poles. Sister chromatids remain attached at their centromeres.
-
Anaphase II
- Sister Chromatid Separation: In meiosis II, similar to mitosis, sister chromatids are separated and move to opposite poles, resulting in the formation of four haploid cells from the original diploid cell.

Clinical Significance
Chromosomal Disorders
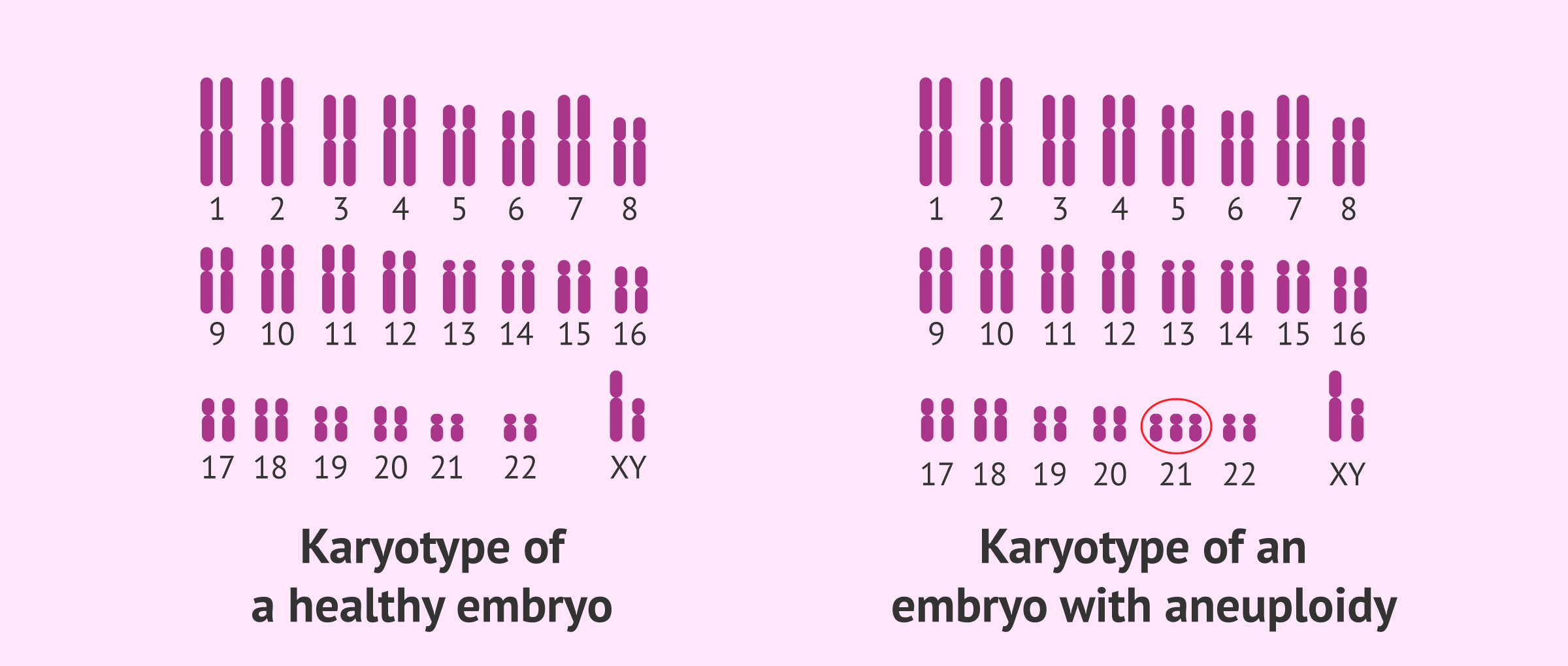
- Aneuploidy: Errors during anaphase, such as nondisjunction (failure of chromatids or homologous chromosomes to separate), can lead to aneuploidy. This condition, where cells have an abnormal number of chromosomes, is implicated in disorders like Down syndrome (trisomy 21).
Cancer
- Chromosomal Instability (CIN): Faulty regulation of anaphase can result in CIN, a hallmark of many cancers. CIN leads to an uneven distribution of chromosomes in daughter cells, contributing to tumor progression and resistance to therapy.
Therapeutic Targets
- Mitotic Inhibitors: Drugs that target microtubule dynamics (e.g., taxanes and vinca alkaloids) disrupt anaphase by preventing proper spindle function, thereby inhibiting the proliferation of cancer cells.
Research and Future Directions
Research into anaphase focuses on understanding the molecular mechanisms that govern chromatid separation and spindle dynamics:
-
Live-Cell Imaging
- Real-Time Observations: Advances in live-cell imaging techniques allow researchers to observe chromosomes movements and spindle dynamics in real-time, providing insights into the mechanistic details of anaphase.
-
Molecular Dissection
- Protein Interactions and Modifications: Investigating the interactions and post-translational modifications of proteins involved in anaphase regulation helps elucidate the precise control mechanisms.
-
Gene Editing and Model Systems
- CRISPR/Cas9: Gene editing tools enable the study of specific genes and proteins involved in anaphase, facilitating the identification of new regulatory factors and potential therapeutic targets.
- Model Organisms: Studies in model organisms like yeast, Drosophila, and mice contribute to our understanding of conserved anaphase mechanisms across different species.
Anaphase FAQ
Anaphase A involves the poleward movement of sister chromatids toward spindle poles, driven by the depolymerization of kinetochore microtubules and the activity of motor proteins, such as dynein and kinesin. Anaphase B is characterized by spindle elongation and separation of spindle poles, mediated by the sliding apart of antiparallel interpolar microtubules and the action of molecular motors, including kinesins and microtubule-associated proteins.
Anaphase onset is regulated by the anaphase-promoting complex/cyclosome (APC/C), a multi-subunit E3 ubiquitin ligase. APC/C promotes the degradation of anaphase inhibitors, such as securin and cyclin B, leading to the activation of separase and cyclin-dependent kinase 1 (Cdk1). Separase cleaves the cohesin complex, allowing sister chromatids to separate, while Cdk1 inactivation triggers mitotic exit. Regulation of APC/C activity involves phosphorylation, ubiquitination, and binding of regulatory proteins, including Cdc20 and Cdh1.
The fidelity of chromosome segregation during anaphase is maintained through multiple mechanisms:
-
Spindle Assembly Checkpoint (SAC): The SAC ensures that all chromosomes are properly attached and bi-oriented before anaphase onset, preventing premature segregation.
-
Cohesin Cleavage: The timely and regulated cleavage of cohesin complexes by separase ensures that sister chromatids are only separated when all are correctly attached and aligned.
-
Microtubule Dynamics: Proper regulation of microtubule dynamics ensures that chromosomes move accurately towards opposite poles. Kinetochore microtubules shorten, and polar microtubules elongate in a controlled manner.
-
Motor Proteins: Dynein and kinesin motor proteins generate forces that drive chromatid movement and spindle elongation, ensuring efficient and accurate segregation.
-
Error Correction Mechanisms: Aurora B kinase and other regulatory proteins detect and correct improper attachments by destabilizing incorrect kinetochore-microtubule interactions, allowing reattachment and ensuring correct segregation.
Anaphase can be divided into two distinct phases, anaphase A and anaphase B, each with specific functions and regulatory mechanisms:
-
Anaphase A:
- Chromatid Movement: Anaphase A is characterized by the movement of sister chromatids towards opposite spindle poles, driven by the shortening of kinetochore microtubules.
- Regulation: Motor proteins such as dynein and the depolymerization of kinetochore microtubules facilitate this process. Microtubule-associated proteins (MAPs) and the regulation of microtubule dynamics are critical.
-
Anaphase B:
- Spindle Elongation: Anaphase B involves the elongation of the mitotic spindle, driven by the sliding of overlapping polar microtubules and the pushing apart of spindle poles.
- Regulation: Motor proteins such as kinesin-5 and dynein, along with the polymerization of polar microtubules, contribute to spindle elongation. Coordination between motor proteins and microtubule dynamics ensures efficient spindle elongation.
-
Interplay: Anaphase A and B can occur simultaneously or sequentially, depending on the cell type and organism. The transition between these phases is regulated by changes in motor protein activity and microtubule dynamics.
Defects in anaphase regulation can lead to chromosome missegregation, aneuploidy, and genomic instability, which are hallmarks of cancer and developmental disorders. Aneuploidy, the gain or loss of chromosomes, disrupts cellular homeostasis, alters gene dosage, and promotes tumorigenesis. Dysregulation of anaphase mechanisms, such as APC/C dysfunction or SAC impairment, can result in mitotic catastrophe, cell death, or the generation of oncogenic mutations. Understanding the molecular basis of anaphase regulation is essential for developing strategies to prevent chromosomes instability-related diseases and improve cancer therapy.
Conclusion
Anaphase is a pivotal phase of mitosis and meiosis characterized by the separation and movement of sister chromatids or homologous chromosomes to opposite poles of the cell. This phase ensures accurate chromosome segregation and genetic stability. The regulation of anaphase involves a complex interplay of molecular mechanisms, including the spindle assembly checkpoint, APC/C activation, and the action of motor proteins. Errors in anaphase can lead to chromosomal disorders and contribute to cancer progression. Advances in research continue to uncover the intricacies of anaphase, offering potential avenues for therapeutic intervention in diseases associated with mitotic dysregulation.
You can read these article on :
Anaphase A : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5372008/
Anaphase B : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8406420/
Metaphase To Anaphase Transion : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2150685/