
Telophase: The Final Phase of Mitosis
Telophase is the concluding stage of mitosis, during which the separated chromosomes reach the opposite poles of the cell and begin to de-condense, transitioning back into their less condensed chromatin state. This phase is crucial for the reformation of the nuclear envelope around each set of daughter chromosomes, ultimately leading to the physical division of the cell in cytokinesis. Telophase ensures that each daughter cell inherits an identical set of chromosomes, completing the process of mitosis.
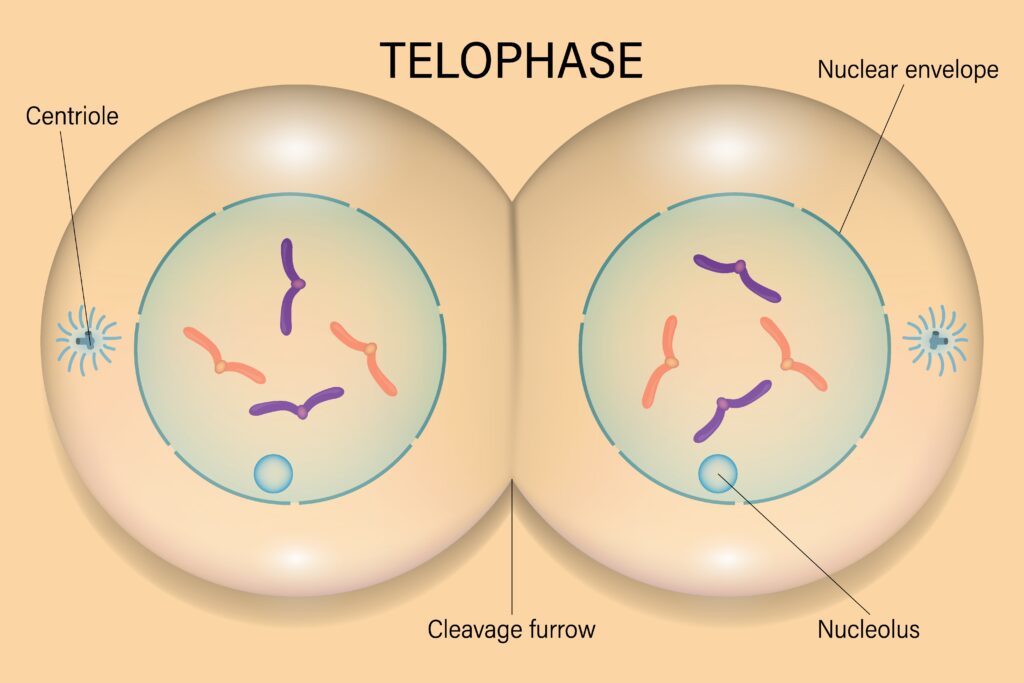
Key Events in Telophase
Chromosome De-condensation
- Transition to Chromatin: Once the chromosomes reach the poles, they begin to de-condense, reverting to the loosely packed chromatin state that is characteristic of interphase. This de-condensation facilitates the resumption of normal cellular activities, such as transcription.
Nuclear Envelope Reformation
- Nuclear Envelope Assembly: Membrane vesicles and components of the nuclear envelope begin to reassemble around each set of chromatids. This process involves the reformation of the nuclear lamina, a network of intermediate filaments that provides structural support to the nucleus.
- Import of Nuclear Proteins: Nuclear pore complexes are re-established, allowing for the selective import of nuclear proteins and the re-establishment of nuclear-cytoplasmic transport.
Nucleolus Reassembly
- Nucleolar Formation: The nucleolus, which disassembles during prophase, begins to reappear in telophase as regions of ribosomal RNA (rRNA) gene transcription resume within the reformed nucleus. The nucleolus is essential for ribosome biogenesis.
Spindle Disassembly
- Spindle Microtubule Breakdown: The mitotic spindle, which facilitated the separation of chromosomes, disassembles during telophase. Microtubules are depolymerized, and tubulin subunits are recycled for use in future cellular processes.
Molecular Mechanisms Regulating Telophase
Regulation by Protein Phosphatases
- Dephosphorylation Events: The transition from metaphase to telophase is regulated by protein phosphatases, such as PP1 and PP2A, which dephosphorylate key mitotic substrates. Dephosphorylation of nuclear lamins, for instance, is critical for the reassembly of the nuclear envelope.

Role of Ran GTPase
-
Nuclear Envelope Formation: Ran GTPase, a key regulator of nuclear transport, plays a crucial role in nuclear envelope reformation. Ran-GTP promotes the fusion of nuclear envelope vesicles around the chromatids.

Mitotic Exit Network (MEN) and NoCut Pathway
- Mitotic Exit: In yeast, the Mitotic Exit Network (MEN) ensures that telophase events proceed correctly, coordinating the inactivation of mitotic kinases and promoting cytokinesis. Similarly, the NoCut pathway monitors the completion of chromosome segregation before cytokinesis can proceed.
Telophase in Meiosis
Telophase occurs in both meiosis I and meiosis II:
-
Telophase I
- Nuclear Reformation: During meiosis I, nuclear envelopes may reform around the separated homologous chromosomes, although this process can vary between species. The cell then proceeds to meiosis II without a significant interphase.
-
Telophase II
- Final Separation: In meiosis II, telophase results in the formation of four haploid daughter cells, each with a unique combination of genetic material. Nuclear envelopes reform around the separated chromatids, completing the meiotic division.
Clinical Significance
-
Chromosomal Disorders
- Errors in Nuclear Reformation: Defects in the processes of nuclear envelope reformation and chromosome de-condensation can lead to genomic instability and aneuploidy, contributing to developmental disorders and diseases such as cancer.
-
Cancer
- Aberrant Mitotic Exit: Cancer cells often exhibit defects in mitotic exit and cytokinesis, leading to abnormal cell division and tumor progression. Understanding the regulation of telophase can aid in the development of targeted therapies to correct these defects.
-
Nuclear Envelope Diseases
- Laminopathies: Mutations in nuclear lamins, which are critical for nuclear envelope reformation, are associated with a group of genetic disorders known as laminopathies. These disorders can affect muscle function, adipose tissue, and overall cellular integrity.
Research and Future Directions
Research into telophase focuses on elucidating the molecular mechanisms that govern nuclear reformation and chromosome de-condensation:
High-Resolution Imaging
- Visualizing Nuclear Dynamics: Advances in high-resolution microscopy allow researchers to visualize the dynamic events of nuclear envelope reformation and chromatin de-condensation in real-time.
Molecular Dissection
- Protein Interactions: Investigating the interactions and regulatory networks of proteins involved in telophase provides insights into their roles in mitotic exit and nuclear reformation.
Genetic Studies and Model Systems
- Gene Editing: Techniques like CRISPR/Cas9 facilitate the study of specific genes and proteins involved in telophase, revealing their functions and potential as therapeutic targets.
- Model Organisms: Studies in model organisms such as yeast, Drosophila, and mice help uncover conserved mechanisms of telophase regulation.
Telophase FAQ
Chromosome decondensation during telophase is regulated by the removal of histone modifications and the displacement of chromatin remodeling complexes. Enzymes, such as histone deacetylases and demethylases, catalyze the removal of acetyl and methyl groups from histone proteins, promoting chromatin relaxation. Decondensed chromatin allows for the re-establishment of nuclear architecture and the resumption of transcriptional activity, facilitating gene expression in the daughter cells.
During telophase, the nuclear envelope reforms around the decondensing chromosomes, marking the end of mitosis or meiosis. This process involves the recruitment of nuclear pore proteins and nuclear membrane vesicles to the chromatin surface. Regulatory proteins, such as Ran GTPase and membrane fusion factors, coordinate the assembly of the nuclear envelope, ensuring its integrity and functionality.
The transition from anaphase to telophase involves several key molecular events:
-
Mitotic Spindle Disassembly: As chromosomes reach the spindle poles, the mitotic spindle begins to disassemble, driven by the depolymerization of microtubules.
-
Nuclear Envelope Reformation: Vesicles containing nuclear envelope components start to reassemble around the separated chromosome sets. The phosphorylation state of nuclear lamins, regulated by CDKs and phosphatases, plays a crucial role in this process.
-
Chromosome Decondensation: chromosomes begin to decondense, transitioning from their highly condensed mitotic state back to the more relaxed interphase chromatin structure, facilitated by the activity of topoisomerase II and histone-modifying enzymes.
-
Reactivation of Transcription and Nuclear Functions: The reformation of the nuclear envelope and the decondensation of chromatin allow the reactivation of transcription and other nuclear functions that were paused during mitosis.
-
Completion of Cytokinesis: Although cytokinesis begins in late anaphase with the formation of the cleavage furrow, it is completed during telophase. The contractile ring composed of actin and myosin filaments constricts to physically separate the two daughter cells.
Chromosome decondensation during telophase is controlled by several mechanisms:
- Histone Modification: The removal of mitotic phosphorylation marks from histones and the re-acetylation of histones promote a more relaxed chromatin structure.
- Topoisomerase II Activity: Topoisomerase II helps resolve any remaining DNA entanglements, facilitating chromatin relaxation.
- Condensin Complex Dissociation: The dissociation of condensin complexes, which were responsible for chromosome condensation during prophase and metaphase, allows chromatin to decondense.
- Nucleosome Remodeling: ATP-dependent chromatin remodeling complexes, such as SWI/SNF, are involved in repositioning nucleosomes to a configuration typical of interphase chromatin.
- Transcriptional Reactivation: The reactivation of transcription machinery and the beginning of transcriptional activity also contribute to chromatin decondensation, as active transcription is associated with a more open chromatin state.
Defects in telophase can result in cytokinesis failure, chromosome missegregation, and genomic instability, leading to cellular dysfunction and disease pathogenesis. Cytokinesis failure can give rise to binucleated or multinucleated cells, which are associated with cancer progression and developmental disorders. Genomic instability resulting from chromosome segregation errors during telophase can lead to aneuploidy, chromosomal rearrangements, and oncogenic transformation. Understanding the molecular mechanisms of telophase regulation is critical for elucidating disease mechanisms and developing targeted therapies.
Conclusion
Telophase is the final phase of mitosis, characterized by the de-condensation of chromosomes, reformation of the nuclear envelope, and disassembly of the mitotic spindle. These events ensure that each daughter cell inherits a complete and identical set of chromosomes. The regulation of telophase involves dephosphorylation events, the action of Ran GTPase, and pathways like the MEN and NoCut in yeast. Understanding the mechanisms of telophase has significant implications for the study of chromosomal disorders, cancer, and diseases associated with nuclear envelope defects. Ongoing research continues to unravel the complexities of telophase, offering potential avenues for therapeutic intervention in mitotic dysregulation.





