
Metaphase: The Second Stage of Mitosis
Metaphase is a critical phase of mitosis during which chromosomes align at the metaphase plate, a plane equidistant between the two spindle poles. This precise alignment ensures that each daughter cell will receive an identical set of chromosomes when the cell divides. Metaphase follows prometaphase and precedes anaphase, marking a key checkpoint in the cell cycle.
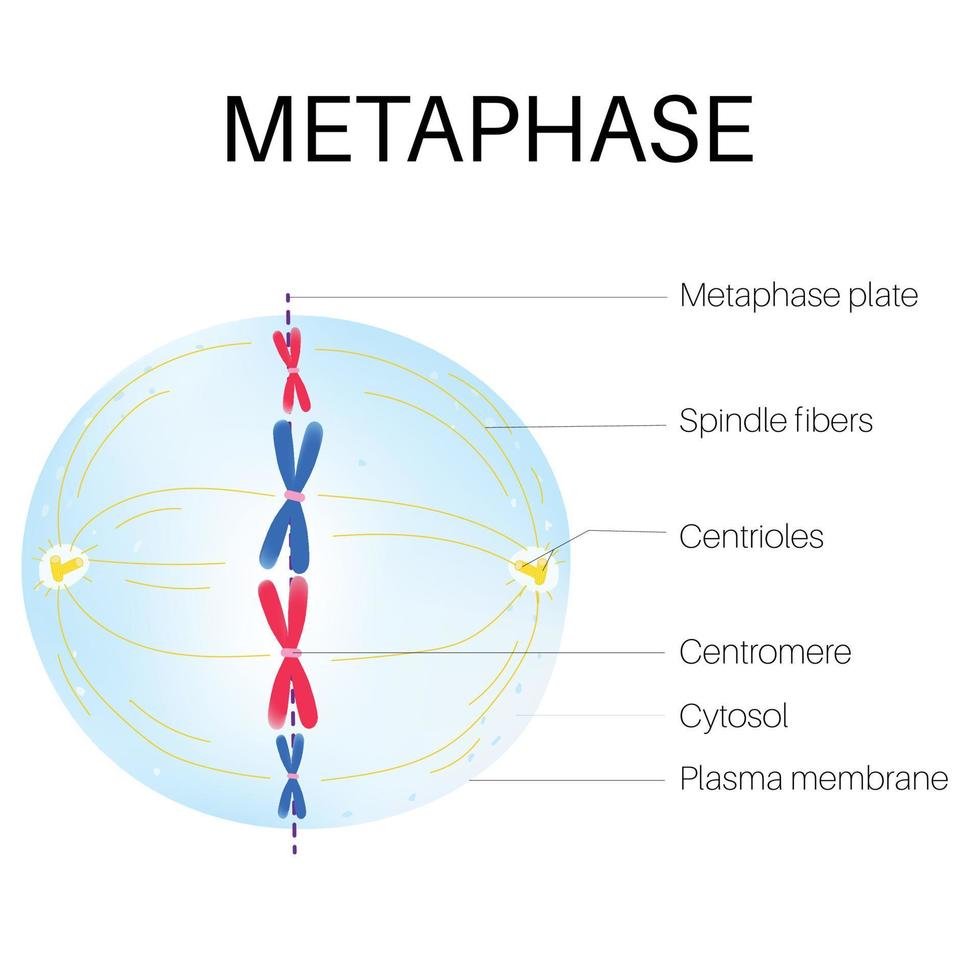
Key Events in Metaphase
Chromosome Alignment
- Formation of Equatorial Plane: During metaphase, chromosomes are aligned at the metaphase plate. This alignment is achieved through the coordinated action of the mitotic spindle, microtubules, and motor proteins.
- Bi-orientation of Chromosomes: Each chromosome's kinetochores are attached to microtubules from opposite spindle poles, ensuring that sister chromatids are poised to be pulled apart in opposite directions during anaphase.

Spindle Assembly and Stability
- Mitotic Spindle: The mitotic spindle is fully formed, consisting of microtubules that extend from centrosomes located at opposite poles of the cell. The spindle microtubules are categorized into kinetochore microtubules, polar microtubules, and astral microtubules.
- Attachment of Kinetochores to Spindle Microtubules: Kinetochores on each chromatid attach to microtubules emanating from opposite spindle poles. This ensures that sister chromatids will be pulled to opposite poles during anaphase.
- Tension Generation: Proper attachment and alignment of chromosomes generate tension at the kinetochores. This tension is crucial for stabilizing microtubule attachments and ensuring accurate chromosome segregation.
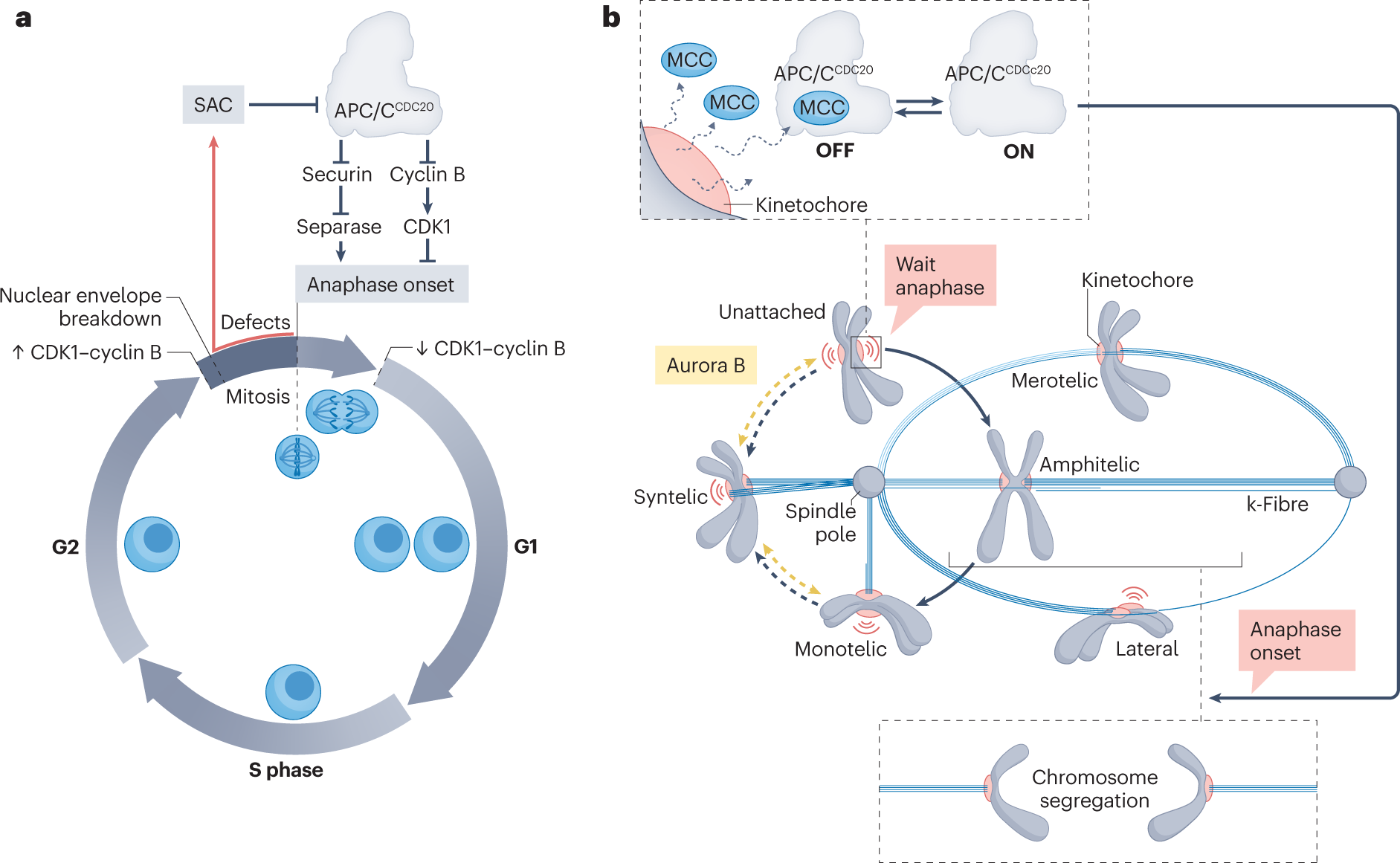
Spindle Assembly Checkpoint (SAC) Activation
- Monitoring of Kinetochore Attachment: The SAC ensures that all chromosomes are properly attached to the spindle microtubules before proceeding to anaphase. This checkpoint prevents the onset of anaphase until all chromosomes are correctly aligned.
- Key Proteins: Proteins such as Mad2, BubR1, and Bub3 are involved in the SAC. These proteins inhibit the anaphase-promoting complex/cyclosome (APC/C) until all kinetochores are properly attached to spindle fibers.
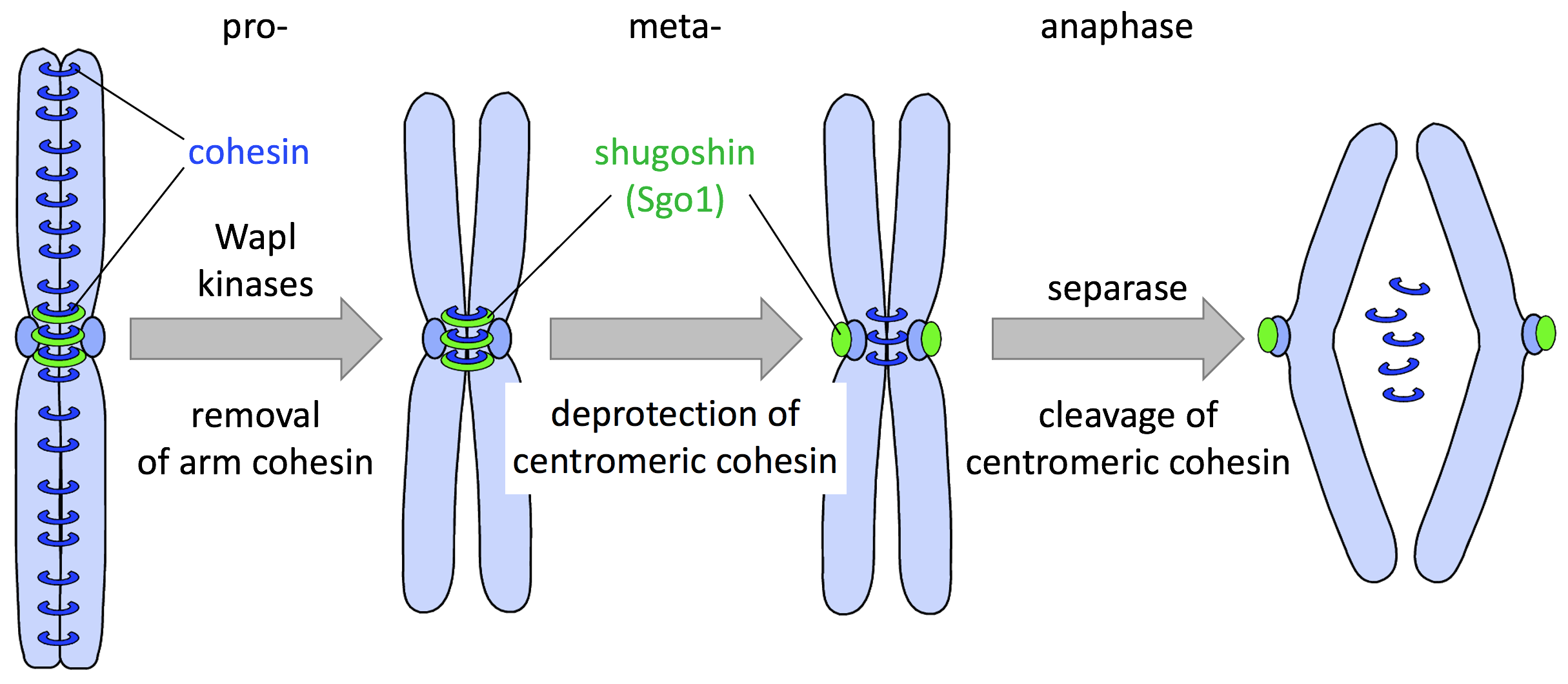
Chromosome Cohesion and Tension Sensing
-
Cohesin Complex: Cohesin proteins maintain the cohesion between sister chromatids until the onset of anaphase.
-
Tension Sensing Mechanisms: Proteins such as Aurora B kinase, part of the chromosomal passenger complex (CPC), sense the tension at kinetochores and facilitate error correction if chromosomes are not properly attached.
Activation of the Anaphase-Promoting Complex/Cyclosome (APC/C)
- Role of APC/C: Once all chromosomes are properly aligned and the SAC is satisfied, APC/C is activated.
- Cdc20: This co-activator of APC/C initiates the ubiquitination and subsequent degradation of securin, which releases separase to cleave cohesin and allow sister chromatid separation.
Microtubule Dynamics and Chromosome Movement
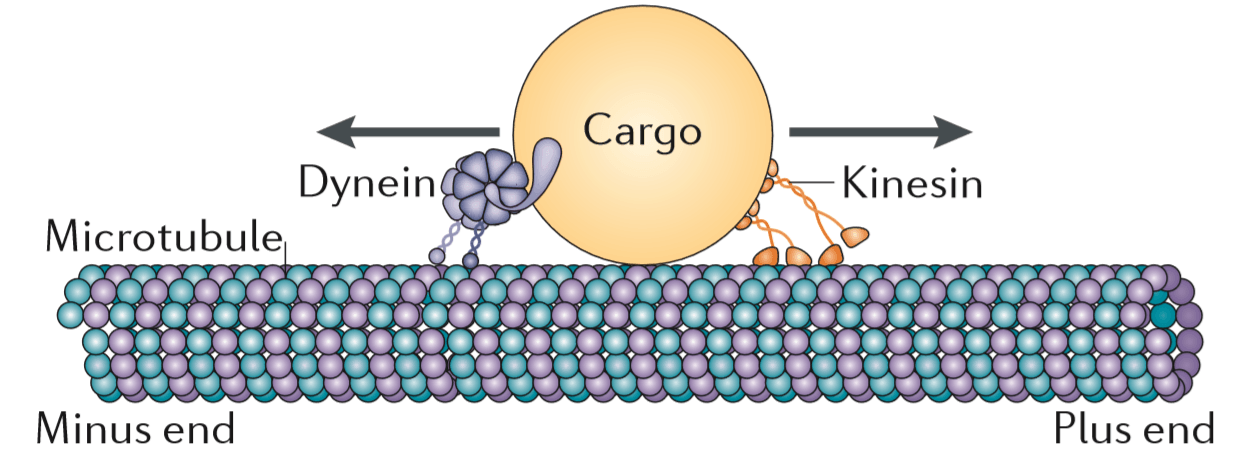
- Stabilization of Microtubules: Microtubules are stabilized through interactions with kinetochores and motor proteins.
- Motor Proteins: Kinesin and dynein motor proteins help position chromosomes at the metaphase plate and maintain tension.
Cytoplasmic Changes
- Reorganization of Cellular Organelles: Organelles such as the Golgi apparatus and endoplasmic reticulum (ER) are positioned to ensure their equal distribution during cytokinesis.
Cell Shape and Cortex Changes
- Cell Cortex Tension: The cell cortex may undergo tension changes, helping to position the spindle apparatus correctly.
Preparation for Anaphase
- Chromosome Checkpoint Clearance: All checkpoints are cleared, ensuring that chromosomes are ready for separation.
- Spindle Pole Integrity: Spindle poles are properly organized to ensure effective chromosome segregation.
These events ensure that chromosomes are correctly positioned and attached to the spindle apparatus, setting the stage for their accurate segregation during anaphase. Proper regulation of these processes is crucial to prevent aneuploidy and maintain genomic stability.
Molecular Mechanisms Regulating Metaphase
Metaphase is regulated by various molecular mechanisms to ensure the accurate and efficient alignment of chromosomes:
Spindle Assembly Checkpoint (SAC)
- Monitoring Chromosome Attachment: The SAC ensures that all chromosomes are correctly attached to spindle microtubules and aligned at the metaphase plate before the cell proceeds to anaphase. The checkpoint proteins, such as Mad2 and BubR1, inhibit the anaphase-promoting complex/cyclosome (APC/C) if any kinetochore is unattached or improperly attached.
- APC/C Activation: Once all chromosomes are correctly aligned and under tension, the SAC is satisfied, leading to the activation of APC/C. APC/C targets securin and cyclin B for degradation, allowing the transition to anaphase.

Kinetochore-Microtubule Attachments
- Dynamic Instability: Microtubules exhibit dynamic instability, alternating between phases of growth and shrinkage. This property is essential for the capture of kinetochores and the correction of improper attachments.
- Motor Proteins: Motor proteins, such as kinesins and dyneins, facilitate the movement and alignment of chromosomes by exerting forces on microtubules. Kinesin-5, for example, helps to separate the spindle poles by cross-linking and sliding polar microtubules.
Chromosome Cohesion
- Cohesin Complex: The cohesin complex holds sister chromatids together from the time of DNA replication until anaphase. During metaphase, cohesin is localized at the centromeres, ensuring that sister chromatids remain connected until the proper time for separation.

Clinical Significance of Metaphase
Chromosomal Disorders
- Aneuploidy: Errors in chromosome alignment and segregation during metaphase can lead to aneuploidy, a condition characterized by an abnormal number of chromosomes. Aneuploidy is associated with various developmental disorders and cancers.
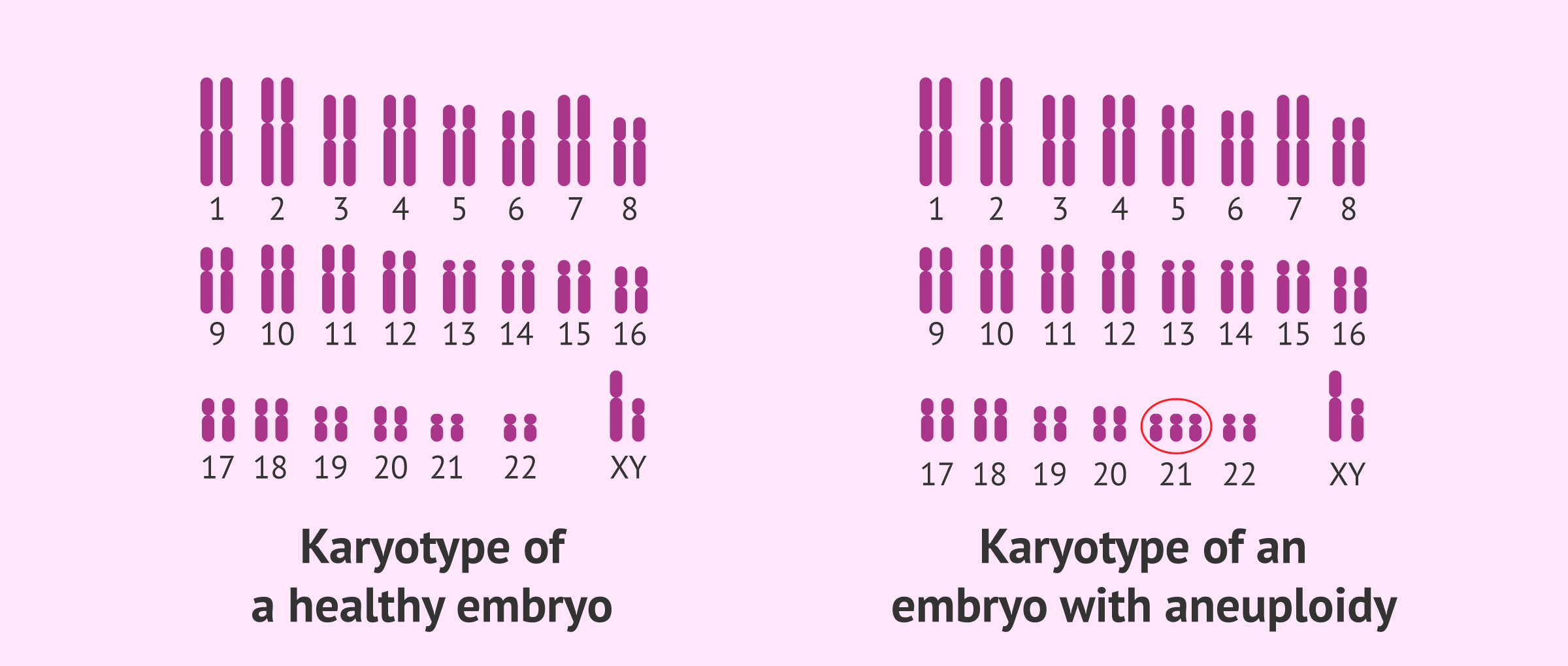
- Down Syndrome: An example of aneuploidy is Down syndrome, caused by the presence of an extra copy of chromosome 21 (trisomy 21), often resulting from non-disjunction during metaphase of meiosis.

Cancer and Therapeutic Targets
- Mitotic Inhibitors: Drugs that target microtubules, such as taxanes and vinca alkaloids, disrupt the mitotic spindle and prevent proper chromosome alignment and segregation. These drugs are used in cancer therapy to inhibit the proliferation of rapidly dividing tumor cells.
- Spindle Checkpoint Defects: Defects in the spindle assembly checkpoint can lead to chromosomal instability (CIN), a hallmark of many cancers. Research into SAC components and their regulation provides insights into cancer progression and potential therapeutic strategies.
Research and Future Directions
Research into metaphase aims to further understand the mechanisms of chromosome alignment and segregation:
Imaging Techniques
- Live-Cell Imaging: Advances in live-cell imaging allow researchers to visualize chromosome dynamics and spindle formation in real-time, providing detailed insights into the processes occurring during metaphase.
- Super-Resolution Microscopy: Techniques such as super-resolution microscopy offer high-resolution views of kinetochore-microtubule attachments and spindle architecture.
Molecular and Genetic Studies
- Protein Interactions: Investigating the interactions between spindle assembly checkpoint proteins, motor proteins, and kinetochores helps elucidate the molecular mechanisms underlying metaphase regulation.
- Gene Editing: Tools like CRISPR/Cas9 enable precise manipulation of genes involved in metaphase, facilitating the study of their functions and roles in chromosome segregation.
Therapeutic Development
- Targeted Therapies: Understanding the molecular details of metaphase regulation can lead to the development of targeted therapies that selectively disrupt mitosis in cancer cells while sparing normal cells.
Metaphase FAQ
Cells can enter a state of metaphase arrest, where progression through metaphase is halted, through various mechanisms. In oocytes, high levels of maturation-promoting factor (MPF), consisting of cyclin-dependent kinase 1 (Cdk1) and cyclin B, maintain metaphase arrest until fertilization. In cancer cells, defects in the spindle assembly checkpoint or alterations in checkpoint signaling pathways can lead to prolonged metaphase arrest, promoting genomic instability and chemotherapy resistance.
Microtubule-associated proteins (MAPs) bind to microtubules and regulate their dynamics, stability, and interactions with chromosomes during metaphase. MAPs such as MAP4, EB1, and CLIP-170 modulate microtubule polymerization, depolymerization, and attachment to kinetochores. By regulating microtubule dynamics, MAPs contribute to the accurate alignment and segregation of chromosomes during metaphase.
Microtubule dynamics are tightly regulated during metaphase to maintain chromosome alignment:
- Dynamic Instability: Microtubules undergo phases of growth and shrinkage, regulated by microtubule-associated proteins (MAPs) and motor proteins.
- Plus-End Tracking Proteins: Proteins such as EB1 and CLIP-170 associate with the growing plus ends of microtubules, stabilizing them and promoting interactions with kinetochores.
- Kinetochore-Microtubule Attachments: Kinetochores control the stability of attached microtubules. Proper attachments are stabilized by tension, while incorrect attachments are destabilized by Aurora B kinase activity.
- Motor Proteins: Kinesin and dynein motor proteins generate forces along microtubules, facilitating chromosome movement and maintaining alignment at the metaphase plate.
- Regulation by MAPs: MAPs such as XMAP215 and MCAK modulate microtubule polymerization and depolymerization rates, ensuring a dynamic yet stable spindle structure that maintains chromosome alignment.
The transition from metaphase to anaphase is triggered by a series of key molecular signals and events:
- SAC Satisfaction: The spindle assembly checkpoint (SAC) must be satisfied, indicating that all chromosomes are properly attached and aligned.
- APC/C Activation: Release of Cdc20 from checkpoint inhibition activates the anaphase-promoting complex/cyclosome (APC/C).
- Ubiquitination of Securin: APC/C ubiquitinates securin, targeting it for proteasomal degradation. Securin inhibits separase; thus, its degradation activates separase.
- Cleavage of Cohesin: Active separase cleaves cohesin complexes that hold sister chromatids together, allowing their separation.
- Cyclin B Degradation: APC/C also targets cyclin B for degradation, leading to the inactivation of CDK1. This inactivation triggers the exit from metaphase and progression into anaphase, as the cell prepares to complete mitosis.
These carefully regulated processes ensure that chromosomes are accurately segregated, maintaining genomic stability in daughter cells.
Advanced imaging techniques, such as high-resolution live-cell microscopy, super-resolution microscopy (e.g., structured illumination microscopy, stochastic optical reconstruction microscopy), and lattice light-sheet microscopy, enable the visualization of metaphase dynamics with unparalleled detail. Computational modeling and simulation approaches, including Monte Carlo simulations and agent-based models, further dissect the molecular mechanisms underlying chromosome alignment and segregation during metaphase. Integration of experimental and computational methods provides comprehensive insights into metaphase regulation and function.
Conclusion
Metaphase is a critical phase of mitosis characterized by the alignment of chromosomes at the metaphase plate and the establishment of bipolar attachments. The accurate segregation of chromosomes during metaphase is essential for genetic stability. The process is tightly regulated by the spindle assembly checkpoint, kinetochore-microtubule interactions, and motor proteins. Errors in metaphase can lead to aneuploidy and are associated with various diseases, including cancer. Advances in imaging, molecular biology, and genetic techniques continue to enhance our understanding of metaphase, offering potential avenues for therapeutic intervention in diseases characterized by mitotic dysregulation.
Read this article on Metaphase: https://www.genome.gov/genetics-glossary/Metaphase
Explore this aricle to expand you idea on Metaphase to Anaphase Transition: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2150685/






