
Prometaphase: A Critical Phase in Mitosis
Prometaphase is a dynamic stage of mitosis that follows prophase and precedes metaphase. During prometaphase, key processes take place that ensure the chromosomes are properly prepared for segregation. These include the complete disassembly of the nuclear envelope, the attachment of chromosomes to the spindle microtubules, and the alignment of chromosomes at the metaphase plate. This phase is essential for the accurate distribution of genetic material to the daughter cells.
Key Events in Prometaphase
Nuclear Envelope Breakdown
- Disassembly of the Nuclear Envelope: During the transition from prophase to prometaphase, the nuclear envelope, which encloses the nucleus, fully disintegrates. This allows the spindle microtubules to access the chromosomes. The disassembly is mediated by the phosphorylation of nuclear lamins and other structural proteins by cyclin-dependent kinases (CDKs).
Spindle Microtubule Attachment
- Kinetochore Formation: Each chromatid of a chromosome forms a kinetochore, a protein structure located at the centromere. Kinetochores serve as attachment points for spindle microtubules.
- Microtubule Capture: Spindle microtubules, emanating from the centrosomes at opposite poles of the cell, attach to the kinetochores. This attachment is crucial for the subsequent alignment and movement of chromosomes.
Chromosome Movement
- Chromosome Congression: Once attached to spindle microtubules, chromosomes begin to move toward the cell’s equatorial plane, or metaphase plate. This process, known as chromosome congression, involves a complex interplay of forces exerted by microtubules and motor proteins, such as dyneins and kinesins.
Bipolar Attachment and Tension
- Bipolar Attachment: For accurate chromosome segregation, each chromosome must be attached to spindle microtubules from both poles (bipolar attachment). This ensures that sister chromatids will be pulled toward opposite poles during anaphase.
- Tension Sensing: Kinetochores can sense tension generated by the bipolar attachment. Proper tension stabilizes microtubule attachments, while lack of tension signals errors that need correction.
Molecular Mechanisms Regulating Prometaphase
Prometaphase is regulated by several molecular mechanisms to ensure fidelity in chromosome segregation:
Cyclin-Dependent Kinases (CDKs)
- Regulation by Cyclin B-CDK1: The activity of cyclin B-CDK1 complex drives the cell from prophase into prometaphase. It phosphorylates key substrates, including nuclear lamins and proteins involved in microtubule dynamics, to promote nuclear envelope breakdown and spindle assembly.
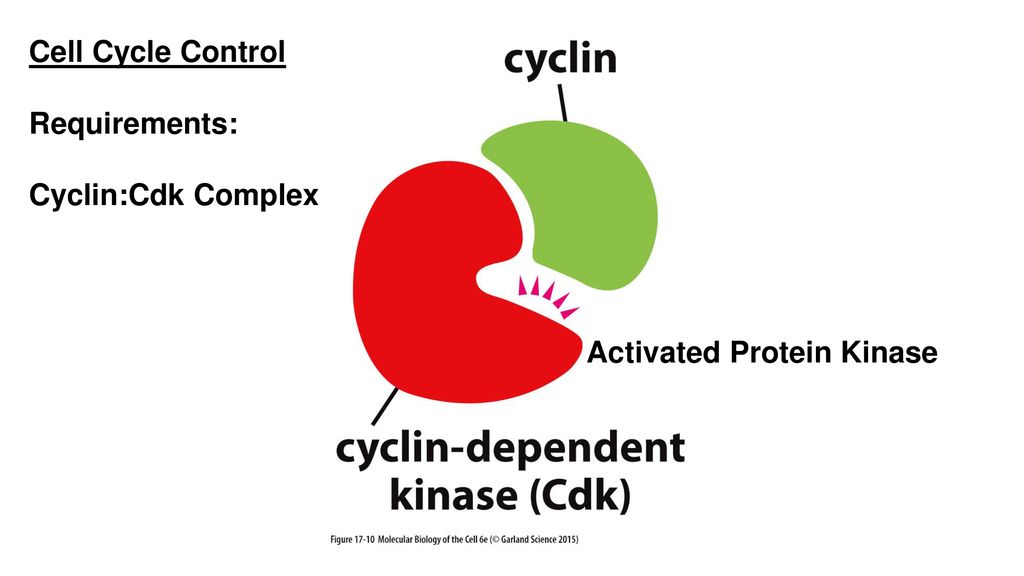
Spindle Assembly Checkpoint (SAC)
- Monitoring Microtubule Attachment: The SAC ensures that all chromosomes are correctly attached to spindle microtubules before proceeding to anaphase. Unattached or improperly attached kinetochores generate signals that delay the onset of anaphase, preventing chromosome missegregation.
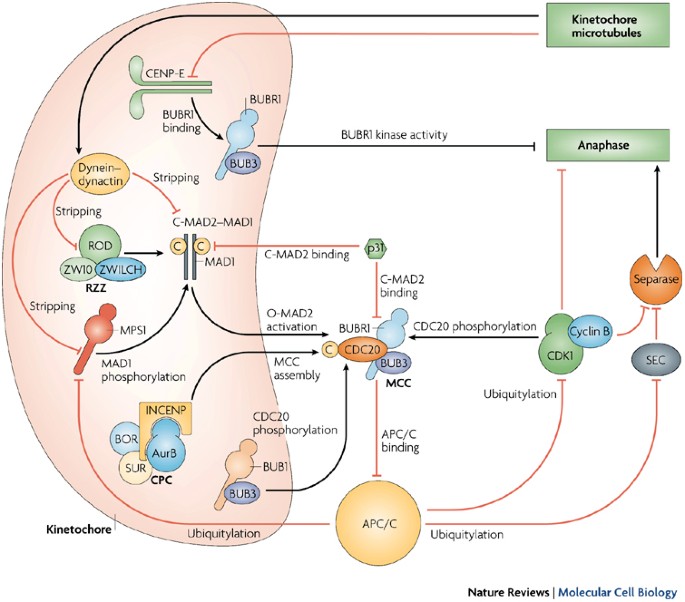
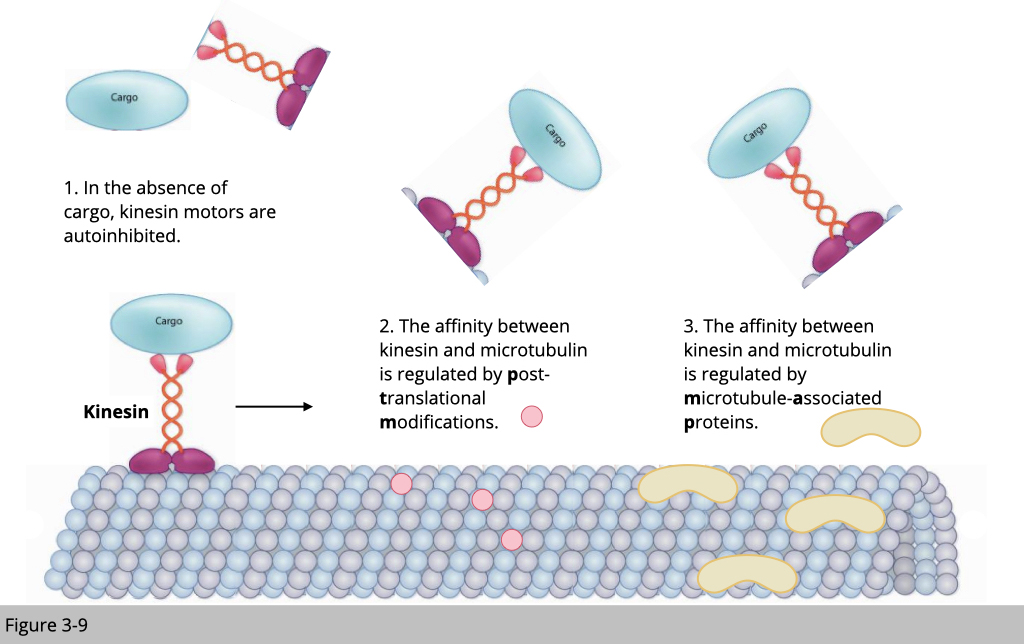
Motor Proteins and Microtubule Dynamics
-
Role of Motor Proteins: Motor proteins, such as dynein and kinesin, play crucial roles in chromosome movement and alignment. They generate forces required for the movement of chromosomes along microtubules.
-
Microtubule Dynamics: The dynamic instability of microtubules, characterized by cycles of growth and shrinkage, is essential for the capture and alignment of chromosomes. Proteins like MCAK (mitotic centromere-associated kinesin) regulate microtubule dynamics at kinetochores.
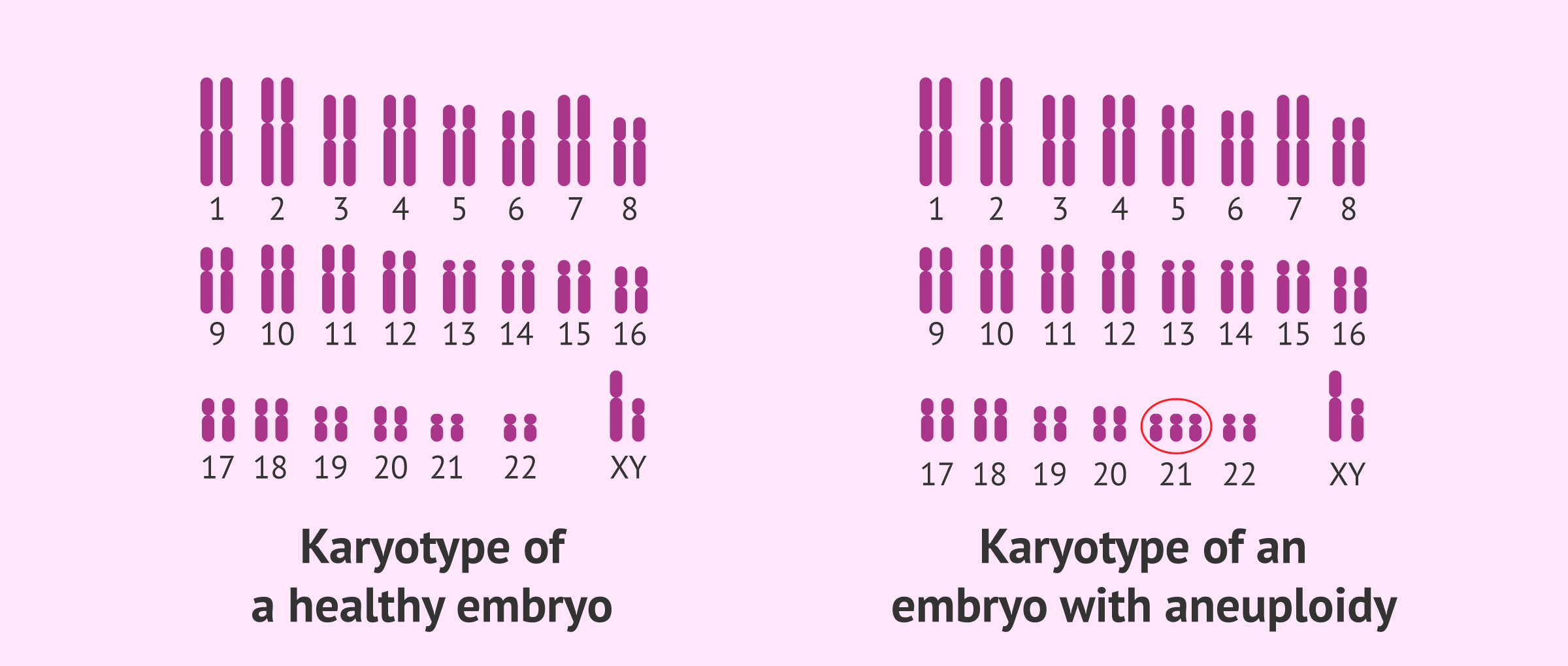
Importance and Clinical Significance
Ensuring Accurate Chromosome Segregation
- Prevention of Aneuploidy: Proper attachment and alignment of chromosomes during prometaphase are critical for preventing aneuploidy, a condition where cells have an abnormal number of chromosomes. Aneuploidy is a hallmark of many cancers and is associated with developmental disorders.
Cancer and Therapeutic Targets
-
Mitotic Errors in Cancer: Errors in prometaphase processes, such as improper kinetochore-microtubule attachments or spindle checkpoint defects, can lead to chromosomal instability in cancer cells.

-
Targeting Prometaphase Mechanisms: Drugs that target microtubule dynamics (e.g., taxanes and vinca alkaloids) or proteins involved in the spindle checkpoint are used in cancer therapy to disrupt mitosis in rapidly dividing cells.
Research and Future Directions
Ongoing research aims to further elucidate the mechanisms governing prometaphase:
High-Resolution Imaging
- Live-Cell Microscopy: Advanced imaging techniques allow researchers to visualize chromosome movements and microtubule dynamics in real-time, providing insights into the processes of kinetochore-microtubule attachment and chromosome congression.
Molecular Dissection of Regulatory Pathways
- Protein Interactions and Modifications: Investigating the interactions and post-translational modifications of proteins involved in prometaphase can reveal new regulatory mechanisms and potential therapeutic targets.
Model Systems and Genetic Studies
- Model Organisms: Studies in model organisms, such as yeast, Drosophila, and mice, help uncover conserved mechanisms of mitotic regulation that can be relevant to human health and disease.
Pro-metaphase FAQ
The formation of stable attachments between kinetochores and microtubules during prometaphase is regulated by a dynamic interplay of motor proteins, kinases, phosphatases, and microtubule-associated proteins. Key regulators include Aurora kinases, which phosphorylate kinetochore proteins to promote microtubule attachment, and protein phosphatases, such as PP1 and PP2A, which counteract kinase activity to stabilize attachments.
The proper attachment of spindle microtubules to kinetochores is ensured through several mechanisms:
- Dynamic Instability of Microtubules: Microtubules constantly undergo phases of growth and shrinkage, increasing the chances of capturing kinetochores.
- Search-and-Capture Model: Microtubules extend from centrosomes in all directions, searching for kinetochores. Once a microtubule attaches to a kinetochore, it stabilizes, reducing dynamic instability at that attachment site.
- Kinetochore-Microtubule Interface: Kinetochore proteins, such as Ndc80 and DAM1/DASH complexes, mediate the attachment of microtubules to kinetochores, ensuring a stable connection.
- Aurora B Kinase Regulation: Aurora B kinase, part of the chromosomal passenger complex (CPC), monitors and corrects improper attachments by phosphorylating kinetochore components to destabilize incorrect attachments, allowing reattachment until proper bi-orientation is achieved.
- Spindle Assembly Checkpoint (SAC): The SAC monitors kinetochore attachment and tension. Unattached or improperly attached kinetochores activate the checkpoint, delaying anaphase onset until all chromosomes are correctly attached and aligned.
Kinetochores generate and respond to mechanical tension to ensure proper chromosome alignment and segregation:
-
Bi-orientation: When kinetochores on sister chromatids are attached to microtubules from opposite spindle poles (bi-orientation), tension is generated as the microtubules pull the kinetochores in opposite directions.
-
Tension Sensing: Tension is sensed by kinetochore proteins, which undergo conformational changes under tension. Proper tension stabilizes kinetochore-microtubule attachments.
-
Aurora B Kinase Role: Aurora B kinase, localized at the inner centromere, phosphorylates kinetochore substrates when there is low tension (indicative of improper attachments). This phosphorylation destabilizes incorrect attachments, allowing for reattachment.
-
Cohesin Complex: The cohesin complex holds sister chromatids together, resisting the pulling forces exerted by microtubules and contributing to the generation of tension.
-
Corrective Mechanisms: If tension is insufficient, error correction mechanisms are activated to detach and reattach microtubules until proper tension and attachment are achieved, ensuring accurate chromosome segregation.
Motor proteins, such as dynein and kinesin, exert force on kinetochore-bound microtubules to drive chromosome movement toward the spindle equator during prometaphase. Dynein, localized at spindle poles, generates poleward pulling forces, while kinesin motors contribute to chromosome congression by mediating chromosome movements away from the poles. The balance of forces generated by motor proteins and microtubule dynamics is critical for proper chromosome alignment.
Microtubules exhibit dynamic instability, characterized by phases of polymerization and depolymerization, which drive spindle assembly and chromosome movements during prometaphase. Regulators of microtubule dynamics, including microtubule-associated proteins (MAPs) and motor proteins, control the growth, shrinkage, and stability of microtubules. Microtubule dynamics are essential for capturing and aligning chromosomes at the metaphase plate and for generating forces required for chromosome segregation.
Conclusion
Prometaphase is a critical phase of mitosis that ensures the accurate attachment and alignment of chromosomes for subsequent segregation. It involves the breakdown of the nuclear envelope, formation of kinetochores, attachment of spindle microtubules, and the movement of chromosomes to the metaphase plate. Proper regulation of prometaphase is essential to prevent chromosomal instability and aneuploidy. Understanding the molecular mechanisms and regulatory pathways of prometaphase has significant implications for cancer biology and the development of therapeutic strategies. Advances in imaging and molecular biology continue to enhance our knowledge of this vital cellular process.
Read this article to get a better idea on A prometaphase mechanism of securin destruction is essential for meiotic progression in mouse oocytes





