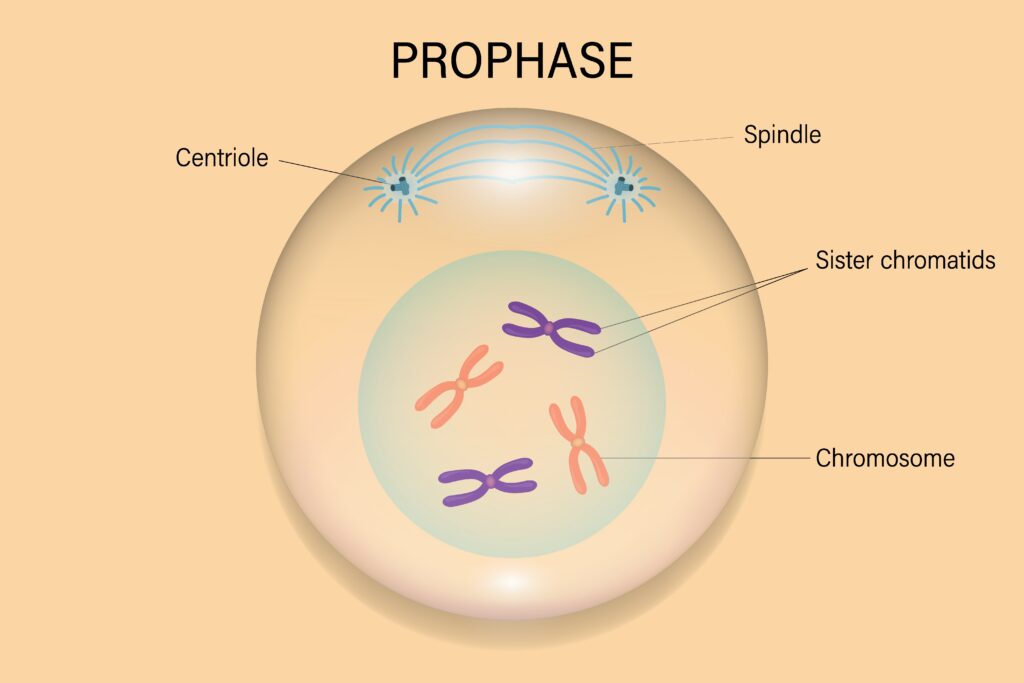
Prophase: The First Stage of Mitosis
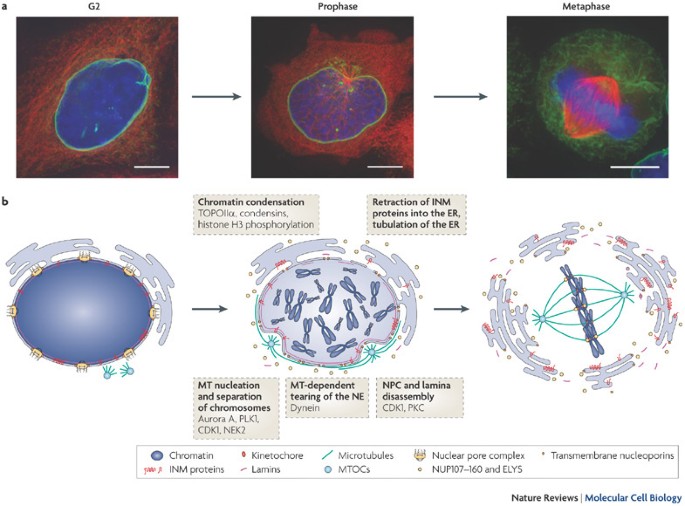
Prophase marks the beginning of mitosis, setting the stage for the accurate segregation of chromosomes into two daughter cells. It is characterized by a series of structural and biochemical changes that prepare the cell for the subsequent stages of mitosis. During prophase, chromosomes condense, the mitotic spindle begins to form, and the nuclear envelope starts to disassemble.
Key Events in Prophase
Chromosome Condensation
-
Formation of Distinct Chromatids: Each chromosome consists of two sister chromatids held together at the centromere. These chromatids become more distinguishable as condensation progresses.
-
Condensation Process: chromosomes, which were duplicated during the S phase, condense into tightly coiled structures that become visible under a light microscope. Each chromosome now consists of two identical sister chromatids joined at a region called the centromere.
-
Role of Condensins: Condensin complexes are essential for chromosome condensation. These protein complexes facilitate the compaction of chromatin into discrete chromosomes, making them easier to segregate later in mitosis.
Mitotic Spindle Formation
-
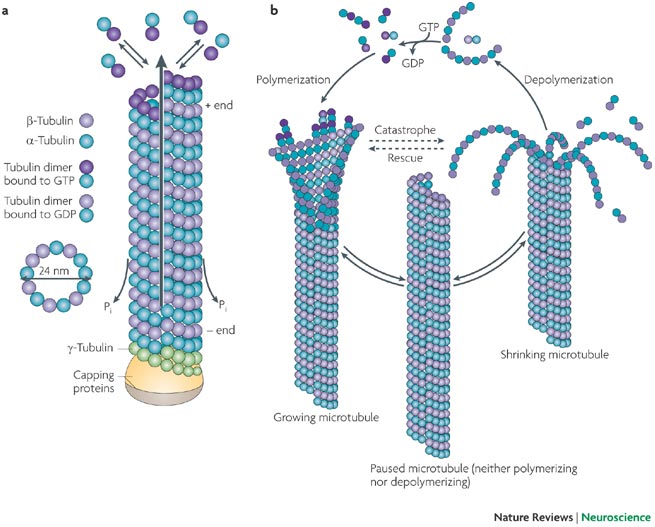
Centrosome Duplication and Migration: The centrosomes, which were duplicated during the S phase, begin to migrate to opposite poles of the cell. Centrosomes are the main microtubule-organizing centers and play a critical role in spindle formation.Each centrosome acts as a microtubule-organizing center (MTOC).

-
Microtubule Polymerization: Microtubules start to polymerize from the centrosomes, forming the mitotic spindle. These microtubules will later attach to the kinetochores on the chromosomes.The spindle apparatus is essential for chromosome movement and segregation during mitosis.
Nuclear Envelope Breakdown
-
Phosphorylation of Nuclear Lamins: The nuclear envelope disassembles as a result of phosphorylation of nuclear lamins by mitotic kinases such as CDK1-cyclin B. This disassembly allows spindle microtubules to access the chromosomes.
-
Nuclear Lamina Disassembly: The nuclear envelope, which encloses the nucleus, begins to disassemble. This process is initiated by the phosphorylation of nuclear lamins, the structural proteins of the nuclear envelope, by cyclin-dependent kinase 1 (CDK1) in complex with cyclin B.
-
Spindle Microtubule Invasion: The breakdown of the nuclear envelope allows spindle microtubules to access the chromosomes. This sets the stage for the attachment of microtubules to kinetochores, specialized protein structures on the chromosomes.
Formation of Kinetochores
- Kinetochores Assembly: Protein complexes called kinetochores assemble on the centromere regions of the chromosomes. These structures will serve as attachment points for spindle microtubules.
- Maturation of Kinetochores: Kinetochores mature and become capable of capturing microtubules emanating from the centrosomes.
Golgi Apparatus Fragmentation
- Golgi Breakdown: The Golgi apparatus fragments into vesicles and tubules, which are distributed into the daughter cells during cell division.
Cytoskeletal Reorganization
- Actin Cytoskeleton Changes: The actin cytoskeleton undergoes reorganization to accommodate the formation of the mitotic spindle and future cytokinesis.
Activation of M-Cdk (Cyclin-Dependent Kinase 1-Cyclin B Complex)
- Entry into Mitosis: The activation of M-Cdk triggers the entry into mitosis. M-Cdk phosphorylates various substrates involved in chromatin condensation, nuclear envelope breakdown, and spindle assembly.
Chromosome Movement
- Chromosomes Begin to Move: Although chromosome movement is more pronounced in prometaphase, initial movements may begin as microtubules attach to kinetochores and exert pulling forces.
Checkpoints and Quality Control
- Spindle Assembly Checkpoint (SAC): While the SAC becomes fully active in prometaphase, initial steps ensure that kinetochores are properly attached to microtubules before proceeding to metaphase.
Golgi and ER Reorganization
- Endoplasmic Reticulum (ER) Changes: The ER undergoes reorganization, and its network becomes fragmented, ensuring that it is properly distributed during cell division.
Nucleolus Disappearance
- Nucleolar Disassembly: The nucleolus, the site of ribosomal RNA synthesis and ribosome assembly, disappears during prophase. This is due to the cessation of rRNA transcription and the dispersal of nucleolar components throughout the nucleoplasm.
These events collectively ensure that the chromosomes are properly prepared and aligned for subsequent separation during metaphase and anaphase. Proper regulation of these processes is crucial for accurate chromosome segregation and the maintenance of genomic stability.
Molecular Regulation of Prophase
The transition from interphase to prophase is tightly regulated by various molecular mechanisms to ensure proper mitotic entry and progression:
-
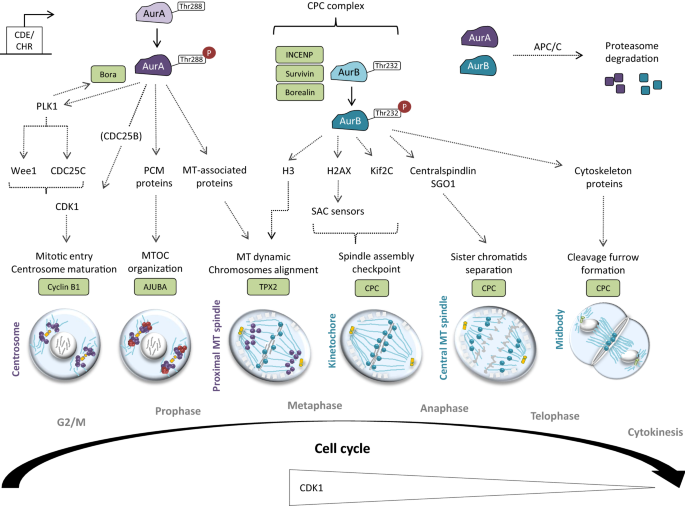
Cyclin B-CDK1 Complex: The activation of the cyclin B-CDK1 complex (also known as maturation-promoting factor, or MPF) is crucial for the initiation of prophase. This complex phosphorylates multiple substrates, leading to the structural changes characteristic of prophase, such as chromosome condensation and nuclear envelope breakdown.

-
Aurora Kinases: Aurora A and Aurora B kinases play important roles in spindle assembly and chromosome condensation. Aurora A is involved in centrosome maturation and spindle formation, while Aurora B is part of the chromosomal passenger complex that regulates chromosome condensation and kinetochore function.
-
Checkpoint Controls: The G2/M checkpoint ensures that cells do not enter mitosis unless DNA replication is complete and any DNA damage is repaired. Key regulators of this checkpoint include ATM/ATR kinases and their downstream effectors CHK1 and CHK2, which inhibit the activation of cyclin B-CDK1 in the presence of DNA damage.

Clinical Significance of Prophase
Defects in the processes and regulation of prophase can lead to chromosomal instability and are often associated with cancer:
-
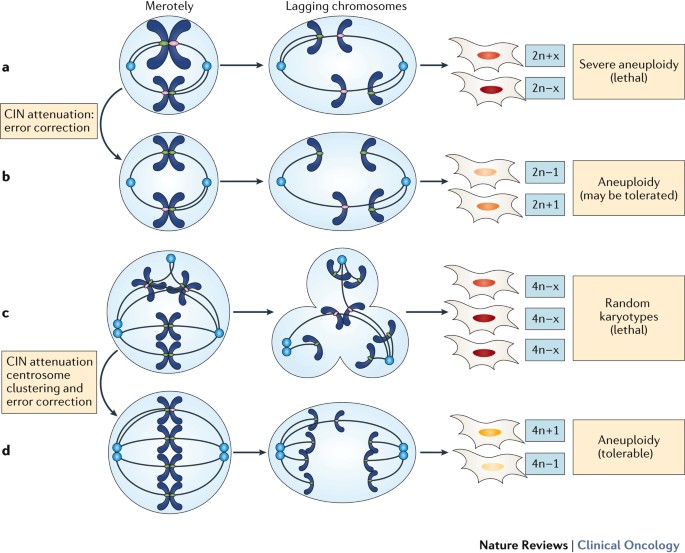
Chromosomal Instability (CIN): Errors in chromosome condensation, spindle formation, or nuclear envelope breakdown can result in improper chromosome segregation, leading to aneuploidy (abnormal number of chromosomes). CIN is a hallmark of many cancers and contributes to tumorigenesis and cancer progression.
-
Cancer Therapeutics: Targeting mitotic regulators is a strategy in cancer therapy. Drugs that inhibit key players in prophase, such as Aurora kinase inhibitors and CDK1 inhibitors, are being investigated for their potential to selectively kill cancer cells by disrupting mitosis.
Research and Future Directions
Ongoing research seeks to deepen our understanding of prophase and its regulation:
- Mechanistic Studies: Advanced imaging techniques and biochemical assays are used to study the dynamic changes in chromatin structure, spindle formation, and nuclear envelope dynamics during prophase.
- Regulatory Networks: Investigating the signaling pathways and protein interactions that regulate prophase will provide insights into how cells coordinate the complex events of mitosis.
- Therapeutic Targets: Identifying novel regulators of prophase and their roles in cancer can lead to the development of new therapeutic targets and strategies to treat mitotic dysregulation in diseases.
Prophase FAQ
The mitotic or meiotic spindle undergoes dynamic changes during Prophase, driven by the assembly and disassembly of microtubules and the movement of motor proteins. Motor proteins, such as dynein and kinesin, generate force to position the spindle poles and align chromosomes. Regulatory proteins, including Ran GTPase and kinetochore-associated proteins, control microtubule dynamics and attachment to chromosomes.
The condensation of chromatin into chromosomes during Prophase is orchestrated by a complex interplay of histone modifications, chromatin remodeling complexes, and protein condensates. Key players include condensins, which are ATP-dependent motor proteins that promote chromatin compaction, and histone H1, which stabilizes higher-order chromatin structures. Various post-translational modifications of histones, such as phosphorylation and acetylation, also regulate chromatin condensation.
During prophase, the centrosomes play a critical role in organizing the mitotic spindle apparatus, which is essential for chromosome segregation. The centrosomes, which duplicate during the S phase, begin to migrate to opposite poles of the cell. Microtubules nucleate from the centrosomes, forming the bipolar spindle apparatus. This spindle is composed of three types of microtubules: astral microtubules, which anchor the spindle poles to the cell cortex; kinetochore microtubules, which attach to the kinetochores on chromosomes; and interpolar microtubules, which interact with microtubules from the opposite pole, stabilizing the spindle. The assembly and dynamics of the spindle are regulated by various motor proteins and microtubule-associated proteins, ensuring accurate chromosome alignment and segregation.
Centrosomes play a pivotal role in organizing the mitotic spindle during prophase:
-
Microtubule Nucleation: Centrosomes serve as microtubule-organizing centers (MTOCs), nucleating and anchoring microtubules that form the spindle apparatus.
-
Centrosome Separation: Centrosomes duplicate during the S phase and begin to separate during prophase, moving to opposite poles of the cell. This separation establishes the bipolar spindle structure.
-
Spindle Pole Formation: Each centrosome forms the spindle pole, from which microtubules radiate and interact with chromosomes. Motor proteins, such as dynein and kinesin, facilitate centrosome movement and spindle assembly.
-
Microtubule Dynamics: Centrosomes regulate the dynamic instability of microtubules, stabilizing those that successfully attach to kinetochores while depolymerizing those that do not, ensuring proper spindle formation and chromosome alignment.
Advanced imaging techniques, such as super-resolution microscopy, live-cell imaging, and electron microscopy, allow researchers to visualize the structural and functional dynamics of Prophase with unprecedented detail. Genomic and proteomic approaches, including chromatin immunoprecipitation sequencing (ChIP-seq) and mass spectrometry, enable the identification of protein-DNA interactions and post-translational modifications that regulate Prophase events. Computational modeling and simulation studies further complement experimental approaches to unravel the complexity of Prophase regulation and function.
Conclusion
Prophase is a pivotal stage in mitosis, marked by chromosome condensation, spindle formation, and nuclear envelope breakdown. These events are regulated by a network of proteins and signaling pathways that ensure the accurate segregation of chromosomes. Understanding the molecular mechanisms and regulation of prophase is crucial for insights into cell division, genomic stability, and the development of targeted therapies for diseases characterized by mitotic dysregulation.
Read this mini-article on Prophase to summarize the main idea https://www.encyclopedia.com/plants-and-animals/zoology-and-veterinary-medicine/zoology-general/prophase