
Unraveling the Mystery of Alpha Particles: A Comprehensive Exploration
In the realm of particle physics, certain entities stand out for their remarkable properties and profound implications for our understanding of the universe. Among these, alpha particles hold a significant place. These particles, consisting of two protons and two neutrons bound together, possess unique characteristics that have captivated scientists for decades. In this article, we delve into the intricacies of alpha particles, exploring their discovery, properties, sources, and diverse applications across various scientific domains.
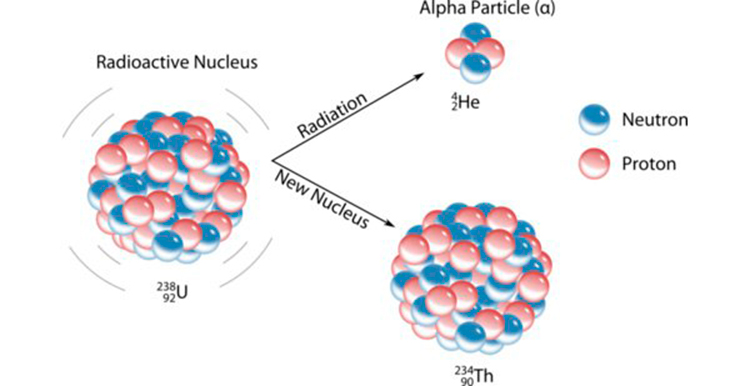
Discovery and Origin
The journey of alpha particles began in the early 20th century when scientists were unraveling the mysteries of radioactivity. In 1899, Ernest Rutherford, a pioneering physicist, coined the term "alpha particle" to describe the positively charged particles emitted by certain radioactive materials. The name "alpha" was chosen to denote the first letter of the Greek alphabet, signifying the prominent position of these particles in the hierarchy of radioactive emissions.He prove it in his experiment which is known as Rutherford's Atomic Model

Further investigations revealed that alpha particles are helium nuclei stripped of their electrons. This discovery elucidated their composition as two protons and two neutrons, identical to the nucleus of a helium-4 atom. The emission of alpha particles occurs during the process of alpha decay, wherein a radioactive nucleus spontaneously emits an alpha particle to transform into a more stable configuration.
Properties of Alpha Particles
Alpha particles exhibit distinct properties that distinguish them from other types of radiation, such as beta and gamma rays.
-
Charge: Alpha particles carry a positive charge equivalent to twice the elementary charge, making them highly ionizing as they interact with matter. This property renders them effective in causing damage to biological tissues and semiconductor materials.
-
Mass: With a mass approximately four times that of a proton, alpha particles possess significant momentum, enabling them to penetrate matter to a limited extent. However, their relatively large mass renders them less penetrating compared to beta and gamma radiation.
-
Speed: Alpha particles typically travel at velocities ranging from 5% to 15% of the speed of light, depending on the energy of the emitted particle and the nature of the radioactive decay process.
-
Range: Due to their high ionization potential and relatively low penetrating power, alpha particles can travel only a short distance in air, typically a few centimeters, before being absorbed.
Sources of Alpha Particles
Alpha particles are produced through various natural and artificial processes, encompassing both terrestrial and extraterrestrial phenomena.
Radioactive Decay: The primary source of alpha particles is the radioactive decay of heavy elements such as uranium, thorium, and radium. During alpha decay, the parent nucleus emits an alpha particle, resulting in the transmutation of the parent atom into a different element.

Cosmic Rays: High-energy alpha particles constitute a component of cosmic rays, which originate from sources beyond the solar system. These cosmic alpha particles, though relatively rare compared to other cosmic ray particles, contribute to the radiation environment of the Earth and the universe at large.
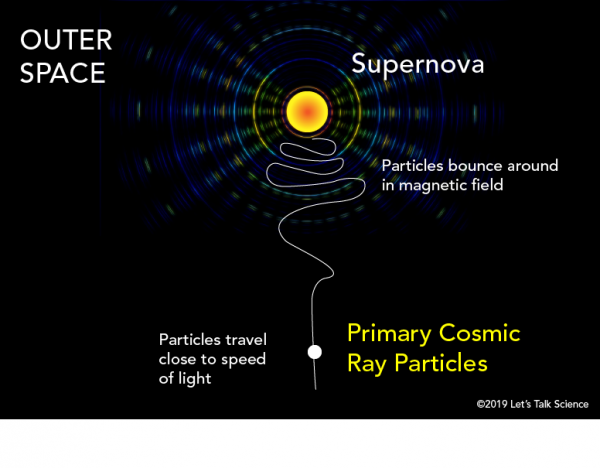
Nuclear Reactions: In nuclear reactions, alpha particles are often emitted as reaction byproducts or projectiles. These reactions occur in nuclear reactors, particle accelerators, and astrophysical environments, contributing to both fundamental research and practical applications.
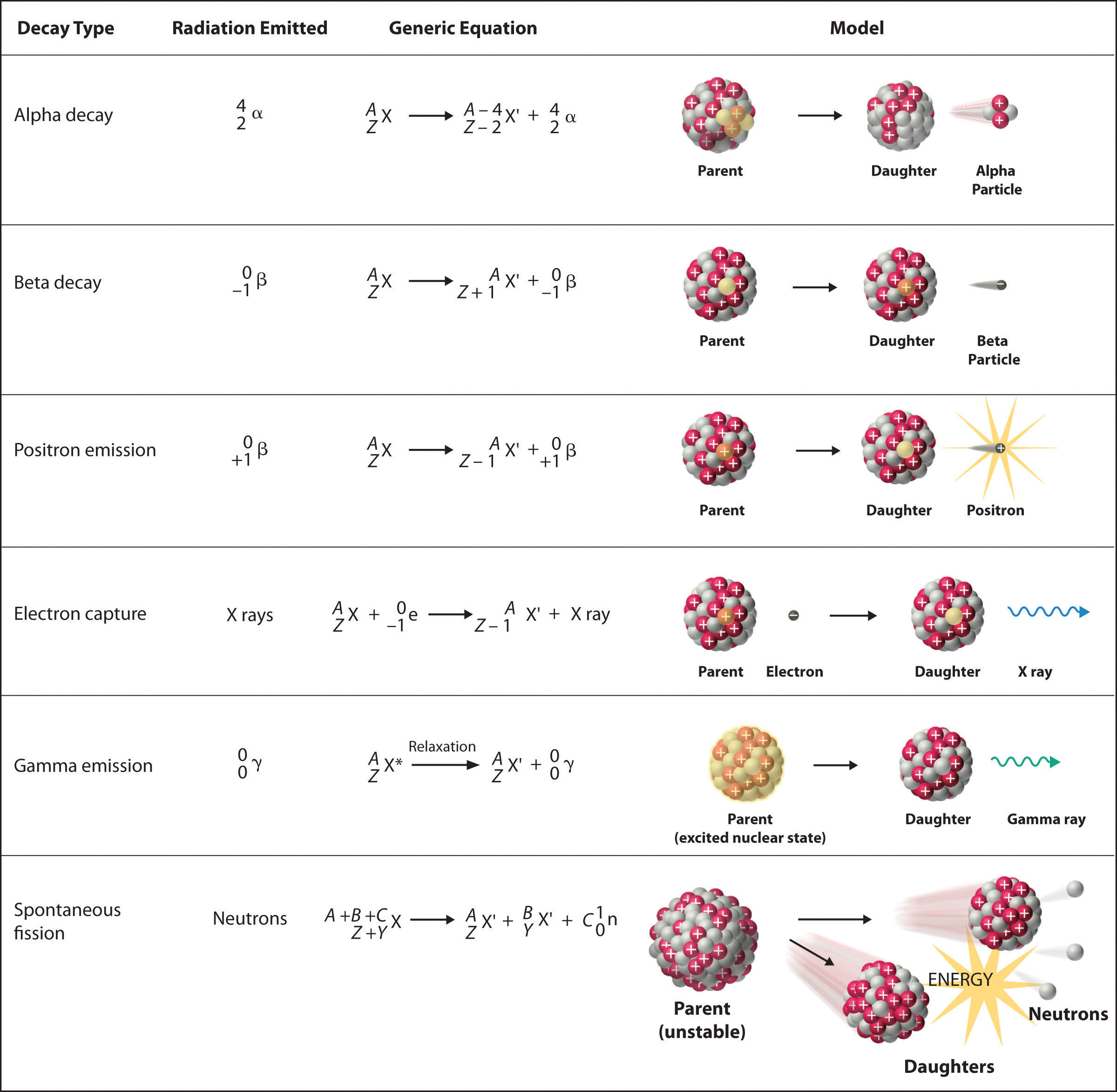
Applications in Science and Technology
The unique properties of alpha particles have led to their utilization across diverse scientific and technological domains, ranging from fundamental research to industrial applications.
-
Radiometric Dating: Alpha decay serves as the basis for radiometric dating techniques, such as uranium-lead dating and potassium-argon dating, enabling the determination of the age of geological formations and archaeological artifacts.
-
Smoke Detectors: The ionizing ability of alpha particles finds practical application in smoke detectors, where a small amount of radioactive material, typically americium-241, emits alpha particles to ionize air molecules, triggering an electrical signal in the presence of smoke.
-
Nuclear Energy: Alpha-emitting isotopes play a crucial role in nuclear power generation, serving as fuel sources in certain types of nuclear reactors. Uranium-235 and plutonium-239, which undergo alpha decay, release energy that is harnessed to generate electricity.
-
Medical Diagnostics and Therapy: In medicine, alpha-emitting isotopes are employed in diagnostic imaging and cancer therapy. Radioisotopes such as radium-223 and actinium-225 emit alpha particles, selectively targeting cancer cells while minimizing damage to surrounding healthy tissue.
Alpha particles FAQ
An alpha particle consists of two protons and two neutrons bound together, making it essentially a helium-4 nucleus. It has a +2 charge due to the presence of two protons and no electrons to balance the positive charge. This configuration gives alpha particles a relatively high mass and a positive electric charge.
Alpha particles interact with matter primarily through ionization and excitation of atoms along their path. Due to their relatively large mass and charge, they have a high likelihood of colliding with other atoms, losing energy quickly. Consequently, they have low penetration power and can be stopped by a few centimeters of air, a sheet of paper, or the outer layer of human skin. This limited penetration power makes them less hazardous externally but potentially dangerous if ingested or inhaled.
In nuclear spectroscopy, alpha particles serve as invaluable probes for studying the structure and properties of atomic nuclei. By bombarding target nuclei with alpha particles, scientists can induce nuclear reactions, leading to the emission of characteristic gamma rays. Analysis of the energy spectra and angular distributions of these gamma rays provides insights into the nuclear structure, including the excitation levels, spin-parity assignments, and decay mechanisms of the target nuclei.
Alpha-emitting radionuclides exhibit unique properties that make them attractive candidates for targeted cancer therapy in radiopharmaceutical development. Due to their high linear energy transfer (LET) and short range in tissue, alpha particles deposit a significant amount of energy along their path, resulting in potent cell-killing effects within tumor cells while sparing surrounding healthy tissues. Researchers are actively exploring the use of alpha-emitting radionuclides, such as Actinium-225 and Radium-223, for the treatment of various malignancies.
The rapid neutron capture process (r-process) is responsible for the formation of approximately half of the elements heavier than iron in the universe. Alpha particles serve as building blocks in the r-process, capturing successive neutrons to form heavier nuclei via rapid neutron capture and subsequent beta decay. The synthesis of heavy elements, such as gold, platinum, and uranium, relies on the intricate interplay between neutron capture, beta decay, and alpha-particle emission during explosive astrophysical events, such as supernovae and neutron star mergers.
Conclusion
Alpha particles, with their distinctive properties and diverse applications, exemplify the intricate interplay between fundamental science and technological innovation. From their role in elucidating the mechanisms of radioactive decay to their practical utility in smoke detectors and medical treatments, alpha particles continue to intrigue and inspire scientists and engineers worldwide. As our understanding of these enigmatic particles deepens, so too does our ability to harness their potential for the betterment of society and the advancement of human knowledge.





