
Atomic Number and Atomic Structure: A Comprehensive Exploration
The atomic number is a fundamental concept in chemistry and physics that plays a pivotal role in defining the structure and properties of an atom. This unique number not only identifies each element but also profoundly influences the arrangement of electrons around the nucleus and the overall atomic behavior. Understanding the relationship between atomic number and atomic structure is key to grasping the principles of chemistry and the periodic table.
What is Atomic Number?
The atomic number Z is defined as the number of protons in the nucleus of an atom. It is a fundamental property that uniquely identifies each element and determines its position in the periodic table. For instance, hydrogen has an atomic number of 1, meaning it has one proton, while carbon has an atomic number of 6, indicating it has six protons.
The Role of Atomic Number in Atomic Structure
Electron Configuration
The atomic number determines the number of electrons in a neutral atom, which in turn dictates the electron configuration. Electrons are arranged in shells or energy levels around the nucleus, following specific rules:
-
Shells and Subshells:
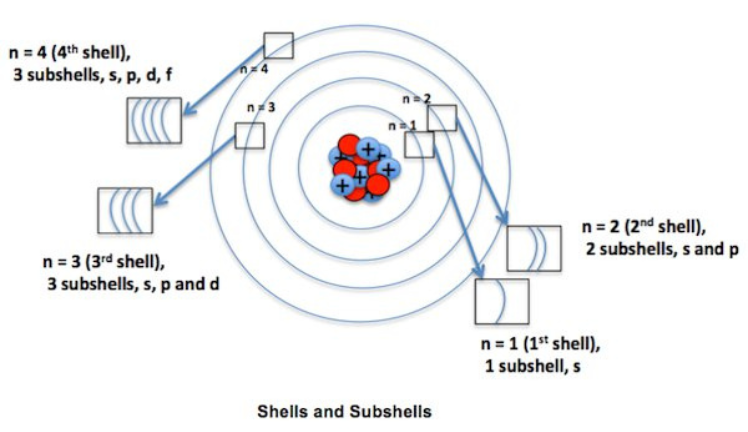 The arrangement of electron atomic numbers around an atom’s nucleus is governed by a structured system of shells and subshells. This organization is crucial for understanding the chemical behavior of elements, predicting bonding patterns, and explaining periodic trends. Let’s delve into the concepts of shells and subshells to uncover how they influence atomic structure.
The arrangement of electron atomic numbers around an atom’s nucleus is governed by a structured system of shells and subshells. This organization is crucial for understanding the chemical behavior of elements, predicting bonding patterns, and explaining periodic trends. Let’s delve into the concepts of shells and subshells to uncover how they influence atomic structure.-
Electron Shells :
Electron shells are the primary energy levels surrounding an atom's nucleus. Each shell can hold a specific maximum number of electrons and is denoted by principal quantum numbers ( n ).
-
Principal Quantum Number ( n ): This number designates the electron shell and determines its energy level. It starts from n = 1 for the closest shell to the nucleus and increases as you move outward.
-
Shell Capacity: The maximum number of electrons that each shell can hold is given by the formula 2n², where n is the principal quantum number.
-
Shell 1 ( n = 1 ): Can hold up to 2 electrons.
-
Shell 2 ( n = 2 ): Can hold up to 8 electrons.
-
Shell 3 ( n = 3 ): Can hold up to 18 electrons.
-
Shell 4 ( n = 4 ): Can hold up to 32 electrons.
-
-
Energy Levels: Electrons in higher shells have higher energy. Electrons fill the lowest energy levels first before occupying higher ones, following the Aufbau principle.
-
-
Subshells :
Within each shell, electrons are further organized into subshells. Subshells represent different types of orbitals within a given shell and are denoted by the angular momentum quantum number ( l ).
-
Types of Subshells: There are four types of subshells, each with a characteristic shape and number of orbitals: Here is a video of atomic orbitals in 3D :
-
s Subshell ( l = 0 ):
- Shape: Spherical.
- Number of Orbitals: 1 orbital.
- Maximum Electrons: 2 electrons.
- Example: 1s, 2s, 3s.
-
p Subshell ( l = 1 ):
- Shape: Dumbbell-shaped.
- Number of Orbitals: 3 orbitals.
- Maximum Electrons: 6 electrons.
- Example: 2p, 3p, 4p.
-
d Subshell ( l = 2 ):
- Shape: Clove-shaped.
- Number of Orbitals: 5 orbitals.
- Maximum Electrons: 10 electrons.
- Example: 3d, 4d, 5d.
-
f Subshell ( l = 3 ):
- Shape: Complex, multi-lobed.
- Number of Orbitals: 7 orbitals.
- Maximum Electrons: 14 electrons.
- Example: 4f, 5f.
-
-
Periodic Trends Related to Shells and Subshells
The arrangement of electrons in shells and subshells directly influences periodic trends:
-
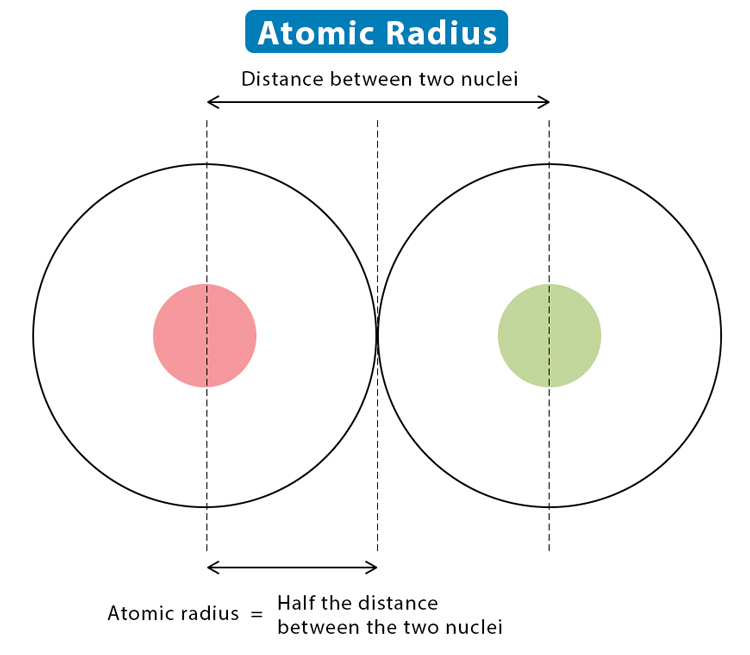
Atomic Radius: Generally decreases across a period as more protons increase the nuclear charge, pulling electrons closer to the nucleus. It increases down a group as additional electron shells are added.
-
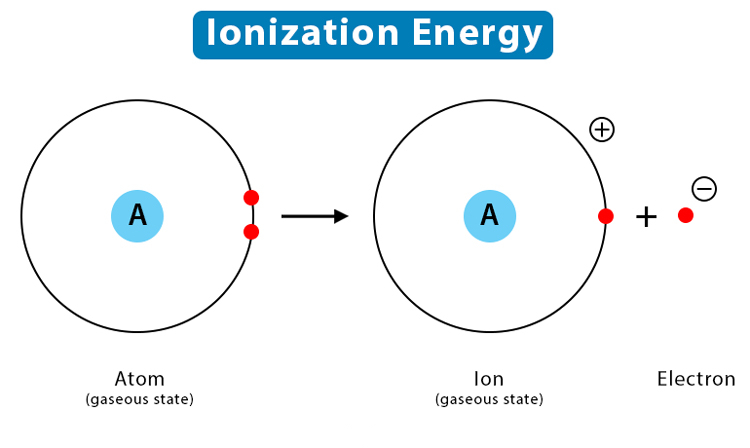
Ionization Energy: Increases across a period due to the increased nuclear charge, which makes it harder to remove electrons. Decreases down a group as electrons are further from the nucleus and more shielded.
-
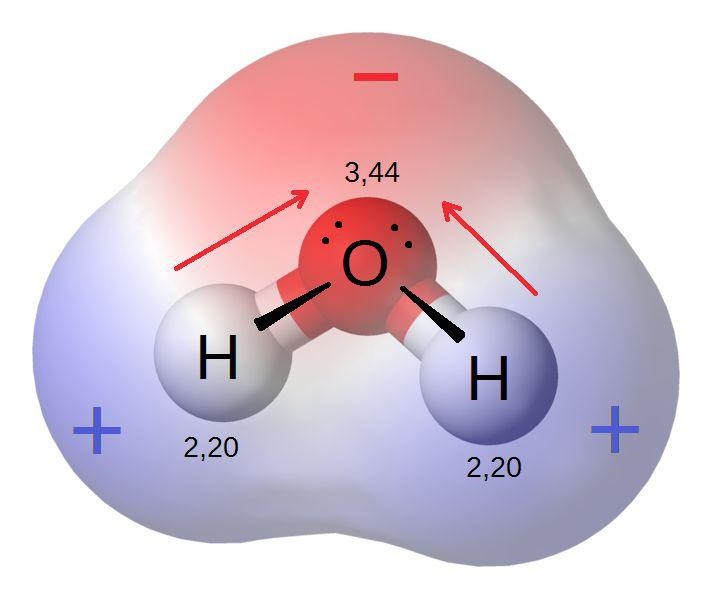
Electronegativity: Tends to increase across a period as atoms attract electrons more strongly with increased nuclear charge. Decreases down a group due to increased distance from the nucleus and electron shielding.
-
-
-
Aufbau Principle: Electrons fill the lowest energy orbitals first before moving to higher ones. For example, for an atom with an atomic number of 6 (carbon), the electron configuration is 1s² 2s² 2p², where electrons fill the 1s and 2s orbitals before occupying the 2p orbitals. Go to this article to understand Aufbau Principle better .

-
Pauli Exclusion Principle and Hund’s Rule: These principles govern the arrangement of electrons in orbitals, ensuring that no two electrons have the same set of quantum numbers and that electrons fill orbitals singly before pairing up.Go to this article to understand Pauli Exclusion Principle and Hund’s Rule better .
Determining Atomic Identity
The atomic number is the primary factor that defines the identity of an element. Elements are distinguished from one another by their atomic numbers, and this number determines the element’s chemical properties and position in the periodic table.
-
Element Identification: The atomic number dictates the number of protons and, in a neutral atom, the number of electrons. For example, an atom with 8 protons is always oxygen, regardless of the number of neutrons.
-
Periodic Table Position: Elements are arranged in the periodic table in ascending order of atomic number. This arrangement reveals periodic trends in element properties, such as atomic radius, ionization energy, and electronegativity.
Impact of Atomic Number on Atomic Structure
Atomic Radius
The atomic number affects the size of an atom. As the atomic number increases across a period (left to right), the atomic radius generally decreases. This occurs because the increasing nuclear charge pulls electrons closer to the nucleus, reducing the atomic size.
-
Across a Period: In Period 2, lithium (Li) has a larger atomic radius compared to fluorine (F). Despite having electrons in the same shell, fluorine’s higher atomic number results in a smaller radius due to a stronger nuclear pull.
-
Down a Group: Moving down a group increases the atomic radius. For instance, in Group 1, potassium (K) has a larger atomic radius than lithium (Li) due to the addition of electron shells.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom in the gaseous state. The atomic number influences ionization energy trends:
-
Across a Period: Ionization energy generally increases across a period. Higher atomic numbers result in increased nuclear charge, making it more difficult to remove electrons.
-
Down a Group: Ionization energy decreases down a group. Despite an increased nuclear charge, additional electron shells and increased shielding reduce the effective nuclear charge felt by the outermost electrons, making them easier to remove.

Electronegativity
Electronegativity measures an atom’s ability to attract electrons in a chemical bond. The atomic number plays a role in determining an element’s electronegativity:
-
Across a Period: Electronegativity increases across a period. The higher nuclear charge attracts electrons more strongly, leading to higher electronegativity.
-
Down a Group: Electronegativity decreases down a group. The increased distance between the nucleus and the bonding electrons, coupled with additional shielding, results in lower electronegativity.

Applications of Atomic Number in Advanced Chemistry
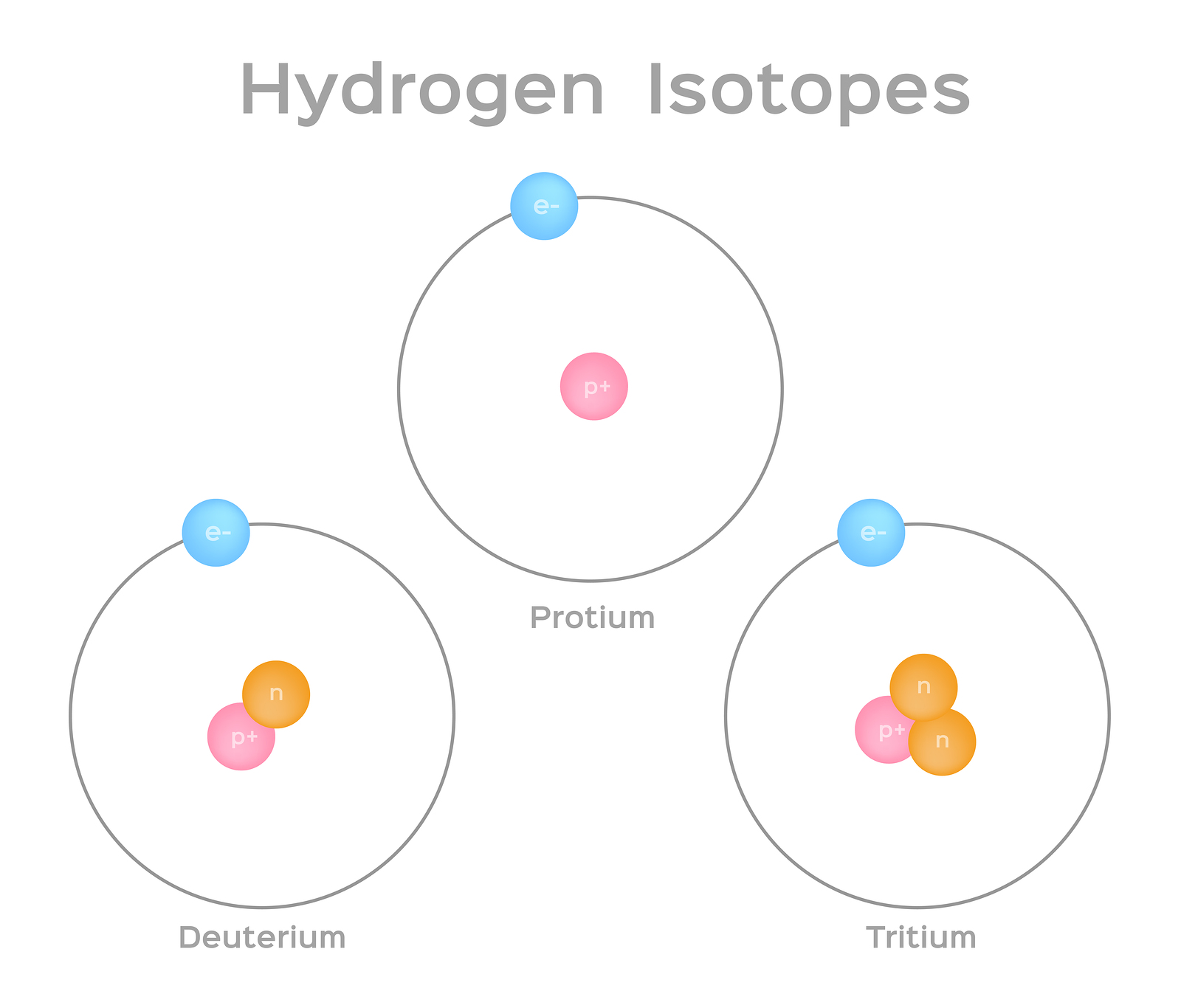
Isotopes and Atomic Number
Isotopes of an element have the same atomic number but different mass numbers due to variations in the number of neutrons. While the atomic number remains constant, isotopes differ in their nuclear stability and properties.
- Stability and Radioactivity: Isotopes with varying numbers of neutrons can be stable or radioactive. Radioactive isotopes undergo decay processes that involve changes in the atomic number, leading to the formation of different elements or isotopes.
Chemical Bonding and Reactivity
Chemical bonding and reactivity are deeply connected to atomic number and atomic structure. The atomic number determines the element's identity and electron configuration, while atomic structure—specifically the arrangement of electrons—governs how atoms interact and form bonds. Here's how they relate:
-
Role of Atomic Number in Chemical Bonding :
- The atomic number (the number of protons in the nucleus) directly determines the number of electrons in a neutral atom. Since chemical bonding involves the interaction of electrons (especially the outermost, or valence electrons), the atomic number dictates the atom's ability to form bonds.
- Atoms with similar numbers of valence electrons (e.g., elements in the same group of the periodic table) tend to exhibit similar bonding behavior. For instance, atoms in Group 1 (alkali metals) have one valence electron, making them highly reactive and prone to forming ionic bonds by losing that electron.
-
Electron Configuration and Bonding :
- The electron configuration—how electrons are distributed across different energy levels and orbitals—determines an atom's chemical reactivity. The valence electrons, located in the outermost shell, are most important in bonding.
- Covalent Bonds: Atoms with incomplete valence shells tend to share electrons to achieve a full outer shell, leading to covalent bonding. For example, carbon (atomic number 6) forms four covalent bonds by sharing its four valence electrons.
- Ionic Bonds: Elements with 1 or 2 electrons in their outer shell (like sodium, atomic number 11) tend to lose these electrons easily, forming positive ions. On the other hand, elements needing a few electrons to fill their outer shell (like chlorine, atomic number 17) tend to gain electrons, forming negative ions. This transfer of electrons results in ionic bonding.
-
Reactivity and Atomic Structure :
- Atomic Size and Reactivity: Atomic size affects reactivity, particularly for metals and non-metals.
- In metals, reactivity increases as atomic size increases (as seen in groups like the alkali metals). For larger atoms, the outer electrons are farther from the nucleus, making them easier to lose, which enhances reactivity.
- In non-metals, reactivity generally decreases as atomic size increases. Smaller atoms, like fluorine (atomic number 9), have a strong ability to attract electrons, making them highly reactive.
- Electronegativity: The ability of an atom to attract shared electrons in a bond is influenced by its atomic structure. The higher the atomic number in a period, the more protons are present in the nucleus, leading to a stronger attraction for electrons (higher electronegativity). For example, fluorine is highly electronegative because of its small size and high nuclear charge.
- Atomic Size and Reactivity: Atomic size affects reactivity, particularly for metals and non-metals.
-
Octet Rule and Chemical Stability :
- Atoms tend to react in ways that allow them to achieve a full valence shell, typically 8 electrons (known as the octet rule). The atomic number determines how many electrons are present and whether the atom is likely to gain, lose, or share electrons to achieve a stable configuration.
- Noble gases (e.g., neon, atomic number 10) have a full outer shell and are chemically inert because they naturally satisfy the octet rule.
- Elements with fewer than 8 valence electrons, such as hydrogen (atomic number 1), are more reactive and tend to form bonds to complete their electron configuration.
- Atoms tend to react in ways that allow them to achieve a full valence shell, typically 8 electrons (known as the octet rule). The atomic number determines how many electrons are present and whether the atom is likely to gain, lose, or share electrons to achieve a stable configuration.
-
Bond Types and Atomic Structure :
- Ionic Bonding: Occurs between atoms with a large difference in electronegativity (typically metals and non-metals). For example, sodium (atomic number 11) readily loses its valence electron, while chlorine (atomic number 17) readily gains one, forming NaCl (table salt).
- Covalent Bonding: Occurs when atoms with similar electronegativities share electrons. For example, two oxygen atoms (atomic number 8) share electrons to form an O₂ molecule with a covalent bond.
- Metallic Bonding: Found in metals, where atoms share a "sea" of delocalized electrons. For example, in iron (atomic number 26), the electrons move freely among the metal ions, giving rise to properties like electrical conductivity and malleability.
-
Periodic Trends in Bonding and Reactivity :
- Across a Period: As you move across a period (left to right) in the periodic table, atoms tend to gain more protons and electrons. Their nuclear charge increases, making them more electronegative and capable of forming stronger covalent bonds. Reactivity decreases for metals (since they prefer to lose electrons) but increases for non-metals.
- Down a Group: As you move down a group, atomic size increases due to additional electron shells. This affects reactivity differently for metals and non-metals:
- Metals: Reactivity increases because it's easier to lose electrons.
- Non-metals: Reactivity decreases because the larger atomic size weakens the attraction to additional electrons.
Synthetic Chemistry and Material Science
In synthetic chemistry, the atomic number guides the creation of new elements and compounds:
-
Elemental Synthesis: By manipulating atomic numbers through nuclear reactions, scientists can create new elements and study their properties.
-
Material Design: The atomic number influences the electronic properties of materials, such as conductivity and magnetism, which are crucial in designing advanced materials for technology and industry.
Chemistry FAQ
The atomic number (the number of protons in the nucleus) uniquely identifies an element and determines its position in the periodic table. Since the number of protons equals the number of electrons in a neutral atom, the atomic number defines the electron configuration of the atom. The arrangement of electrons, particularly in the outermost (valence) shell, dictates how an atom interacts with others, influencing its chemical bonding and reactivity. Elements with similar atomic numbers tend to have similar chemical properties because they have comparable electron configurations. You can learn a bit more about atomic numbers on a seperate article in https://tidewave.net/blog/atomic-number
The atomic number of an element is constant for all isotopes of that element because isotopes have the same number of protons but different numbers of neutrons. For instance, carbon always has an atomic number of 6 (6 protons), but its isotopes, like carbon-12 and carbon-14, have different neutron counts. The atomic number defines the element itself, while the neutron number distinguishes its isotopes. Although isotopes have nearly identical chemical properties, they can differ in physical properties such as mass and radioactivity.
As you move across a period (from left to right) in the periodic table, the atomic number increases, which means more protons are added to the nucleus. This causes the effective nuclear charge (the net positive charge experienced by an electron) to increase as well. Despite the increase in atomic number, electrons are added to the same principal energy level (shell), not to a new, more distant shell. The increased nuclear attraction pulls the electrons closer to the nucleus, resulting in a smaller atomic radius, even though the number of protons and electrons both increase.
Ionization energy is the energy required to remove an electron from a neutral atom in the gas phase. The atomic structure, particularly the electron configuration and nuclear charge, greatly influences this trend. As the atomic number increases across a period, the nuclear charge increases, pulling electrons closer to the nucleus and making them harder to remove, thus increasing ionization energy. However, moving down a group adds electron shells, increasing atomic radius and reducing the pull of the nucleus on the outermost electrons. This makes it easier to remove electrons, thus decreasing ionization energy as you go down a group.
The atomic number plays a crucial role in defining the quantum mechanical model of the atom by influencing the number of electrons that must be accommodated in different orbitals. According to quantum mechanics, electrons occupy discrete energy levels (or shells), and within each shell, there are specific sub-levels and orbitals. The atomic number dictates how many electrons must be placed in these orbitals. The way electrons fill these orbitals, following the Pauli Exclusion Principle and Hund’s Rule leads to specific energy configurations that determine the atom's stability, magnetic properties, and chemical behavior.
Conclusion
The atomic number is a foundational concept that defines the identity, structure, and behavior of atoms. It determines the number of protons in the nucleus, influences electron configuration, and shapes the physical and chemical properties of elements. By understanding the relationship between atomic number and atomic structure, we gain valuable insights into the principles of chemistry, the periodic table, and the behavior of elements in various scientific and practical applications.





