Understanding Atomic Numbers: From Basic Concepts to Advanced Applications
Atomic numbers are fundamental to understanding the structure and behavior of elements in chemistry. Whether you're a beginner studying for O-level exams or an advanced student delving into atomic theory, grasping the concept of atomic numbers is crucial. This article will guide you through the basics and explore more advanced topics, ensuring a comprehensive understanding.
What is an Atomic Number?
The atomic number of an element is the number of protons in the nucleus of an atom of that element. It is denoted by the symbol Z. The atomic number is a unique identifier for each element, meaning that no two elements share the same atomic number.
- Example: The atomic number of hydrogen is 1, which means every hydrogen atom has 1 proton in its nucleus. carbon has an atomic number of 6, indicating 6 protons in each carbon atom.

Why Atomic Numbers Matter
The atomic number is more than a mere count of protons; it’s a gateway to understanding the element’s chemical behavior, its interactions with other elements, and its role in forming compounds. It determines the arrangement of electrons, which in turn influences how atoms bond, react, and interact with light and other forms of energy.
In essence, the atomic number is the essence of an element’s identity, the cornerstone of its chemistry, and the key to unlocking the mysteries of the material world. By grasping the significance of this fundamental number, we gain deeper insight into the intricate dance of atoms that shapes the universe.
Relationship Between Atomic Number and the Periodic Table
The periodic table is a marvel of scientific organization, meticulously arranging elements in a way that reveals patterns in their properties and behaviors. Central to this organization is the atomic number, a unique identifier for each element. Understanding the relationship between atomic number and the periodic table is crucial for grasping the principles of chemistry.
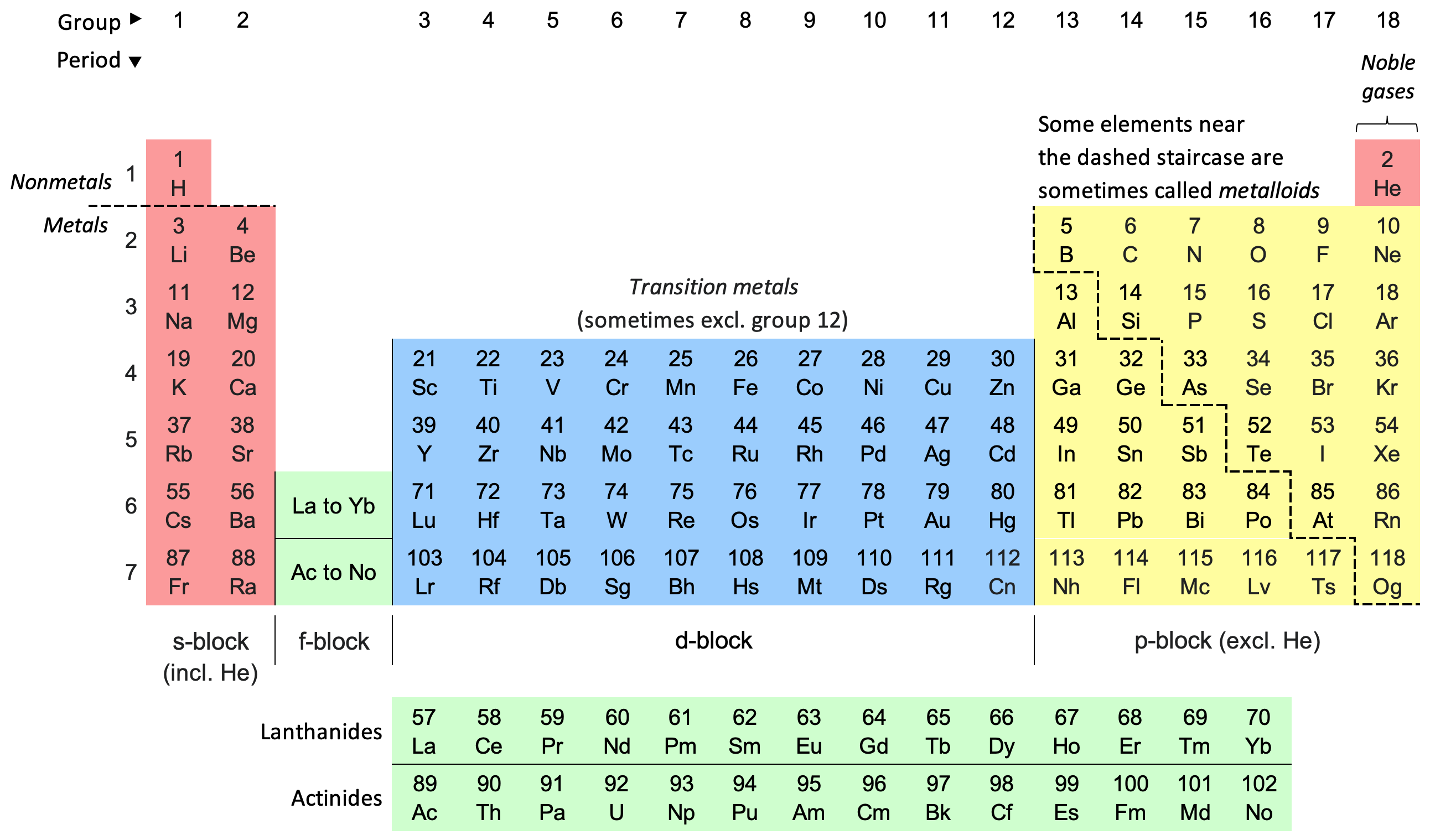
1 . Organizing Elements by Atomic Number
The periodic table arranges elements in order of increasing atomic number, starting from hydrogen with an atomic number of 1 and proceeding sequentially. This arrangement is more than just a numerical sequence; it reflects the underlying principles of atomic structure and chemical behavior.
-
Atomic Number as a Primary Sort: Elements are placed in rows, or periods, based on their increasing atomic number. As you move from left to right across a period, each element has one more proton and one more electron than the element before it.
-
Periodicity: The periodic nature of the table means that elements with similar chemical properties recur at regular intervals. This periodicity is directly related to the repeating patterns in electron configuration that arise from the atomic number.
:max_bytes(150000):strip_icc()/periodicity-58ed43915f9b582c4d8c33e5.jpg)
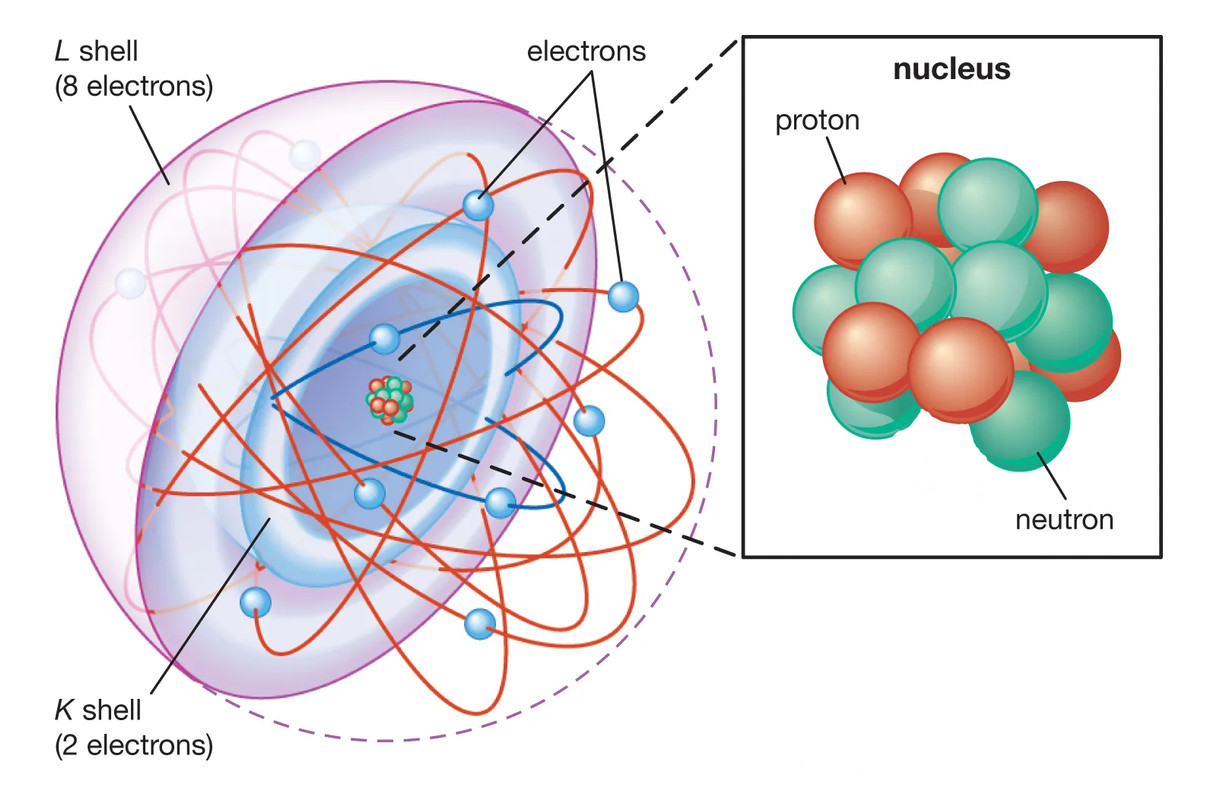
2 . Periods and Electron Shells
The periodic table is organized into rows called periods, which correspond to the number of electron shells surrounding the nucleus of an atom.
-
Shells and Periods: Each period represents the filling of a new electron shell. For example, elements in the first period (like hydrogen and helium) have electrons in the first shell, while those in the second period (like lithium and neon) have electrons in the first and second shells.
-
Period Trends: As you move across a period from left to right, the number of protons and electrons increases, leading to a greater positive charge in the nucleus. This increased nuclear charge pulls electrons closer, affecting properties such as atomic radius and ionization energy.
3 . Groups and Valence Electrons
The columns of the periodic table are called groups or families. Elements in the same group have similar chemical properties due to their identical number of valence electrons, which are electrons in the outermost shell.
-
Group Similarities: For instance, elements in Group 1 (alkali metals) all have one valence electron and exhibit similar reactivity, such as vigorous reactions with water. Similarly, Group 17 (halogens) all have seven valence electrons and are highly reactive nonmetals.
-
Chemical Properties: The atomic number determines the number of electrons and protons, thus influencing the element’s chemical behavior. Elements in the same group have similar chemical properties because their electron configurations are similar, leading to similar bonding and reactivity patterns.
4 . Block Structure and Subshells
The periodic table is also divided into blocks that correspond to the electron subshells being filled: s, p, d, and f blocks.
-
s-Block and p-Block: The s-block (Groups 1 and 2) and p-block (Groups 13 to 18) elements have their outermost electrons in s and p orbitals, respectively. The transition between these blocks occurs as you move from one group to another, reflecting changes in electron configurations.
-
d-Block and f-Block: The d-block (transition metals) and f-block (lanthanides and actinides) involve the filling of d and f orbitals, respectively. The d-block elements are characterized by their unique ability to form multiple oxidation states, while the f-block elements include rare earth metals with specific magnetic and electronic properties.
5 . Periodic Trends Influenced by Atomic Number
The atomic number not only determines an element’s position on the periodic table but also influences various periodic trends:
-
Atomic Radius: As you move across a period from left to right, the atomic radius decreases due to the increasing nuclear charge, which pulls electrons closer. Conversely, as you move down a group, the atomic radius increases because additional electron shells are added.
-
Ionization Energy: Ionization energy generally increases across a period because the increased nuclear charge makes it more difficult to remove an electron. In contrast, ionization energy decreases down a group as additional electron shells reduce the effective nuclear charge experienced by outer electrons.
-
Electronegativity: Electronegativity, or the ability of an atom to attract electrons in a bond, increases across a period and decreases down a group. This trend is a result of changes in atomic number affecting the nuclear charge and electron affinity.
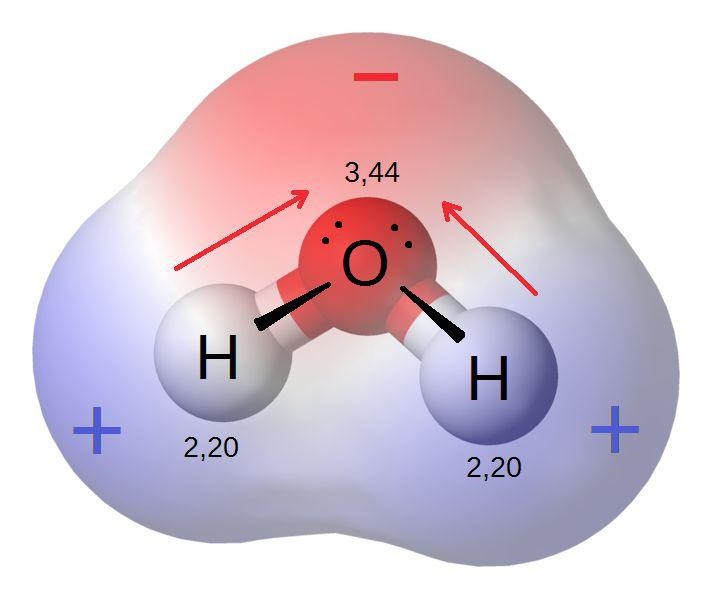
6 . Atomic Number and Element Classification
The atomic number plays a crucial role in classifying elements into categories based on their properties:
-
Metals, Nonmetals, and Metalloids: The periodic table classifies elements into metals, nonmetals, and metalloids based on their properties. Metals (found on the left and center of the table) tend to lose electrons and form positive ions, while nonmetals (found on the right) gain electrons to form negative ions. Metalloids exhibit properties intermediate between metals and nonmetals.
-
Transition and Inner Transition Metals: The transition metals (d-block) and inner transition metals (f-block) are distinguished by their electron configurations and unique chemical properties. These classifications are influenced by the atomic number, which dictates the filling of d and f orbitals.
Atomic Number and Atomic Structure
The atomic number is crucial in determining the overall structure of an atom:
-
Protons and Electrons: In a neutral atom, the number of protons (atomic number) equals the number of electrons. For example, a carbon atom has 6 protons and 6 electrons.
-
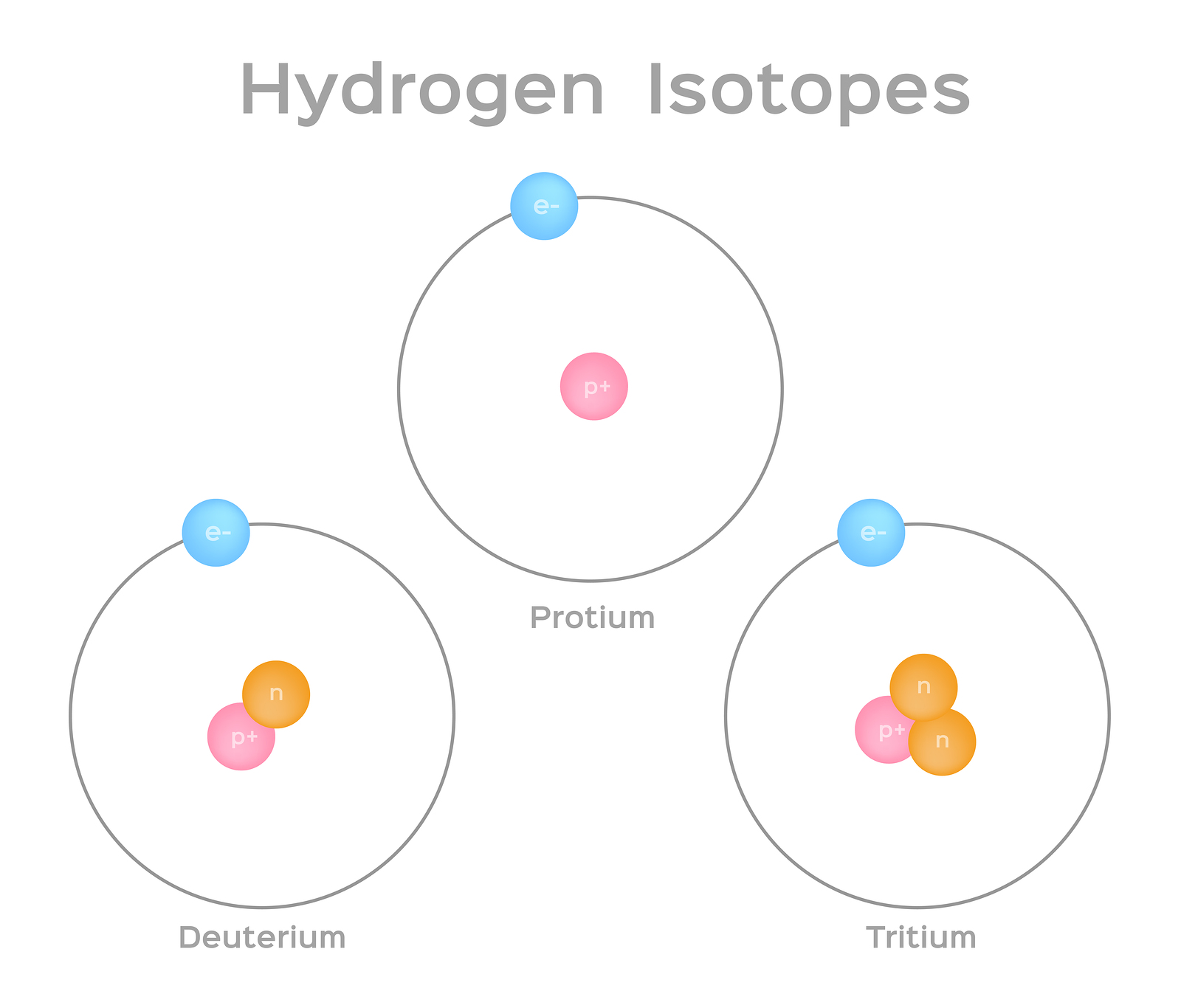
Isotopes: Isotopes are atoms of the same element (same atomic number) but with different numbers of neutrons. For instance, carbon-12 and carbon-14 are isotopes of carbon with atomic number 6 but different mass numbers (12 and 14, respectively).
The Significance of Atomic Numbers
-
Elemental Identity: The atomic number serves as a unique identifier for each element. For example, carbon has an atomic number of 6, meaning every carbon atom contains exactly 6 protons. This unique number differentiates carbon from all other elements.
-
Chemical Behavior: The atomic number determines the number of electrons in a neutral atom, which in turn dictates the element's chemical properties and behavior. For instance, the electron configuration — how electrons are arranged around the nucleus—depends directly on the atomic number.
-
Periodic Table Organization: In the periodic table, elements are arranged by increasing atomic number, which reflects their atomic structure and chemical properties. This arrangement helps predict how elements will interact with one another.
-
Isotopic Variation: While the atomic number remains constant for an element, the number of neutrons can vary, leading to different isotopes. These isotopes have the same atomic number but different atomic masses.
Advanced Understanding of Atomic Numbers
Quantum Mechanical Perspective
From a quantum mechanical viewpoint, the atomic number plays a critical role in defining the electronic structure of an atom.
-
Electron Configuration: The arrangement of electrons around the nucleus of an atom is governed by quantum mechanics. The atomic number determines the number of electrons, which fill the atomic orbitals according to the principles of the Pauli Exclusion Principle , Hund's Rule, and the Aufbau Principle.
- Aufbau Principle: Electrons fill orbitals starting with the lowest energy level.

- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers.
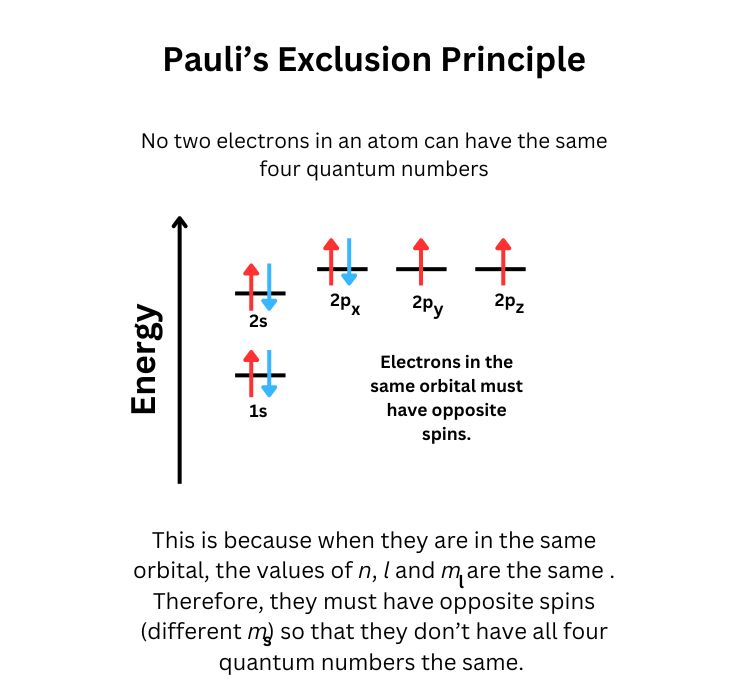
- Hund's Rule: Electrons fill degenerate orbitals singly before pairing up.
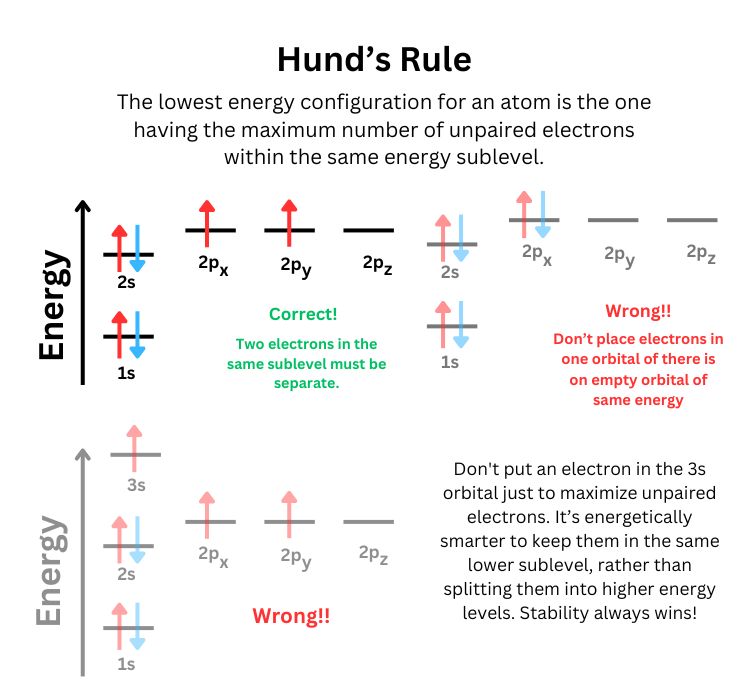
- Aufbau Principle: Electrons fill orbitals starting with the lowest energy level.
-
Energy Levels and Subshells: The atomic number helps define the number of electrons in each energy level (n) and subshell (s, p, d, f), which are described by quantum numbers.
Example: For carbon (Z = 6), the electron configuration is 1s² 2s² 2p², indicating 2 electrons in the 1s orbital, 2 in the 2s orbital, and 2 in the 2p orbitals.
Atomic Number and Periodic Trends
The periodic table is a treasure trove of information about the elements and their properties, organized in a way that reveals fascinating patterns and trends. Central to these trends is the atomic number, which profoundly influences the behavior and characteristics of elements. Let’s explore how atomic number shapes periodic trends, providing insights into the organization of elements and their chemical properties.
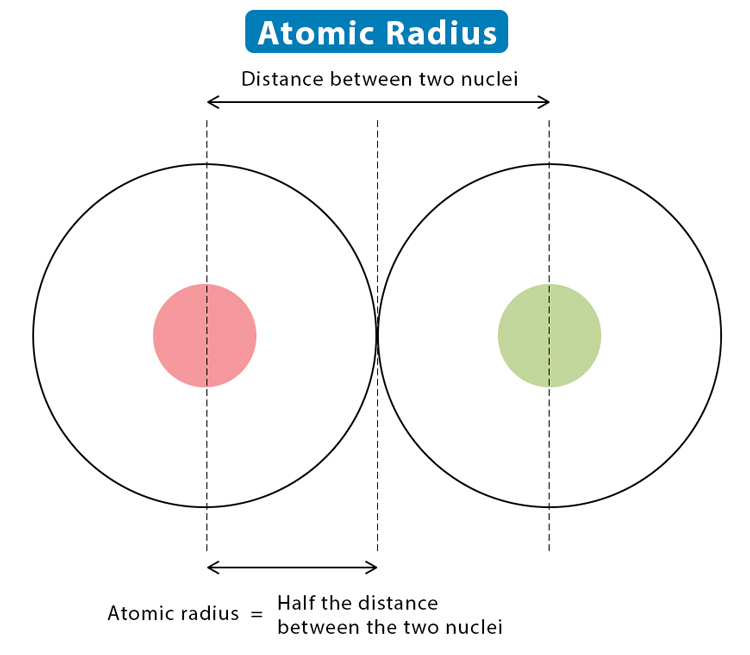
1. Atomic Radius
The atomic radius is a measure of the size of an atom, specifically the distance from the nucleus to the outermost electrons.
-
Trend Across a Period: As you move from left to right across a period, the atomic radius decreases. This decrease occurs because the atomic number increases, resulting in a higher positive charge in the nucleus. This increased nuclear charge attracts electrons more strongly, pulling them closer to the nucleus and thus reducing the atomic radius.
Example: In Period 2, lithium (Li) has a larger atomic radius than fluorine (F). Despite both having electrons in the same shell, fluorine’s higher atomic number leads to a greater effective nuclear charge, drawing electrons closer and making the atom smaller.
-
Trend Down a Group: Moving down a group, the atomic radius increases. This is due to the addition of new electron shells, which outweigh the increase in nuclear charge. Although the nuclear charge is greater, the added electron shells increase the distance between the nucleus and the outermost electrons, resulting in a larger atomic radius.
Example: In Group 1, lithium (Li) has a smaller atomic radius compared to potassium (K), even though both are alkali metals. Potassium has additional electron shells, making it larger despite its higher atomic number.
-
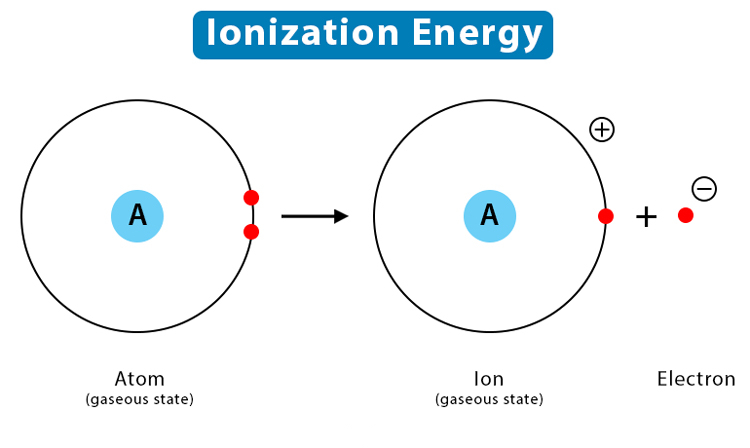
2. Ionization Energy
Ionization energy is the energy required to remove an electron from an atom in its gaseous state.
-
Trend Across a Period: Ionization energy generally increases as you move from left to right across a period. This is because the atomic number increases, leading to a greater nuclear charge which holds electrons more tightly. As a result, more energy is required to overcome the attraction between the nucleus and the electron.
Example: In Period 2, helium (He) has a higher ionization energy compared to lithium (Li). Helium’s increased nuclear charge makes it more difficult to remove an electron.
-
Trend Down a Group: Ionization energy decreases as you move down a group. Although the nuclear charge increases, the additional electron shells create more shielding, reducing the effective nuclear charge felt by the outermost electrons. This means that less energy is required to remove an electron.
Example: In Group 1, cesium (Cs) has a lower ionization energy than lithium (Li). The extra electron shells in cesium decrease the attraction between the nucleus and the outermost electron, making it easier to remove.
3. Electronegativity
Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a chemical bond.
-
Trend Across a Period: Electronegativity increases as you move from left to right across a period. The increasing atomic number means a higher nuclear charge, which enhances the atom’s ability to attract electrons.
Example: In Period 2, fluorine (F) is more electronegative than lithium (Li). Fluorine’s higher nuclear charge enables it to attract electrons more effectively in chemical bonds.
-
Trend Down a Group: Electronegativity decreases as you move down a group. The increased number of electron shells results in more shielding, reducing the nucleus’s ability to attract additional electrons.
Example: In Group 17, iodine (I) has a lower electronegativity compared to fluorine (F), as the larger atomic radius and increased electron shielding in iodine lessen its electron-attracting ability.
4. Electron Affinity
Electron affinity is the energy change that occurs when an atom gains an electron.
-
Trend Across a Period: Electron affinity generally becomes more negative (indicating a higher affinity) as you move from left to right across a period. The increasing atomic number results in a greater effective nuclear charge, which makes it easier for an atom to attract an additional electron.
Example: In Period 2, oxygen (O) has a higher (more negative) electron affinity compared to lithium (Li). Oxygen’s higher nuclear charge makes it more effective at attracting electrons.
-
Trend Down a Group: Electron affinity becomes less negative (indicating a lower affinity) as you move down a group. The added electron shells increase electron shielding and reduce the effective nuclear charge felt by the additional electron.
Example: In Group 17, iodine (I) has a lower electron affinity compared to fluorine (F), as the increased distance between the nucleus and the added electron in iodine diminishes the attraction.
5. Atomic and Ionic Sizes
The size of an atom can also be affected by its ionic state.
-
Cations: When an atom loses one or more electrons to form a positive ion (cation), the resulting ion is typically smaller than the neutral atom. This is due to the reduced electron-electron repulsion and increased effective nuclear charge.
Example: Sodium (Na) loses an electron to form a Na⁺ ion, which is smaller than the neutral Na atom.
-
Anions: When an atom gains one or more electrons to form a negative ion (anion), the resulting ion is typically larger than the neutral atom. This is because the added electrons increase electron-electron repulsion and expand the electron cloud.
Example: Chlorine (Cl) gains an electron to form a Cl⁻ ion, which is larger than the neutral Cl atom.
Nuclear Reactions and Atomic Number
In nuclear chemistry and physics, the atomic number plays a key role in determining the outcomes of nuclear reactions:
-
Nuclear Fission and Fusion: During nuclear reactions, the atomic number can change, leading to the formation of different elements. For example, in nuclear fission, uranium-235 ( Z = 92 ) splits into smaller nuclei, such as barium-141 ( Z = 56 ) and krypton-92 ( Z = 36 ).
-
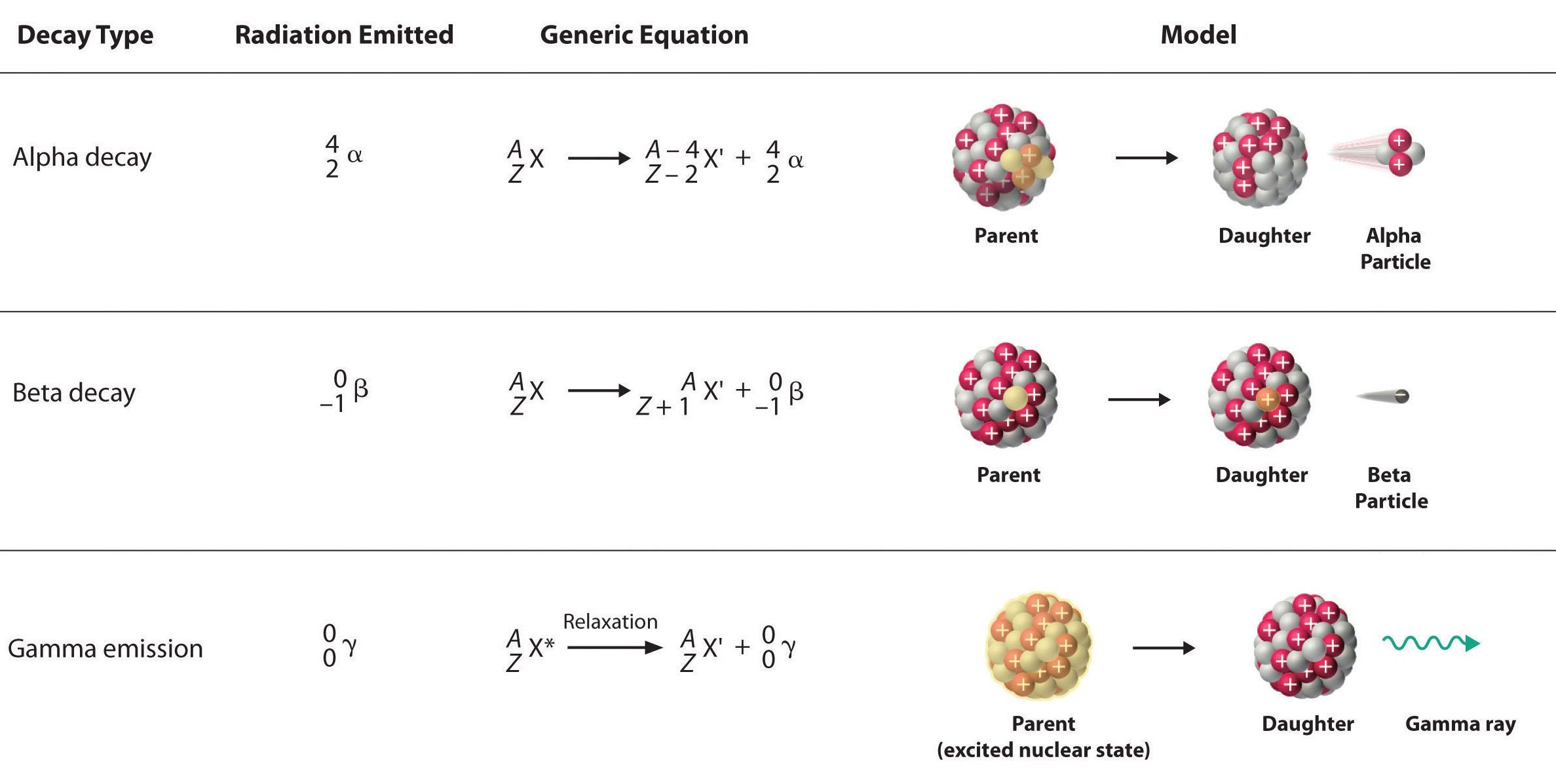
Radioactive Decay: Radioactive decay processes, such as alpha decay (where an atom emits an alpha particle) and beta decay (where a neutron transforms into a proton or vice versa), result in changes in the atomic number of the element.
Atomic Number and Element Synthesis
-
Superheavy Elements: Scientists synthesize elements with very high atomic numbers (greater than 92, uranium) in laboratories. These superheavy elements are often unstable and exist for only fractions of a second before decaying into lighter elements. The atomic number is crucial in these synthesis experiments to determine the identity and stability of the newly created element.
-
Stability and Magic Numbers: The concept of "magic numbers" in nuclear physics refers to certain numbers of protons or neutrons that lead to especially stable nuclei. For example, nuclei with atomic numbers that correspond to magic numbers(like 2, 8, 20) are more stable.
Applications of Atomic Numbers
Chemical Reactions and Stoichiometry
Understanding atomic numbers is essential in predicting the products of chemical reactions and in balancing chemical equations. The atomic number allows chemists to determine how atoms of different elements interact and bond with one another.
Spectroscopy and Atomic Number
In spectroscopy, the atomic number helps in identifying elements by their characteristic emission or absorption spectra. The energy levels of electrons, which are defined by the atomic number, dictate the wavelengths of light that an atom can absorb or emit.
Materials Science and Atomic Number
The atomic number plays a key role in materials science, particularly in understanding the properties of materials, such as conductivity, magnetism, and hardness. For example, elements with higher atomic numbers tend to have higher densities and may exhibit different electrical or thermal conductivities compared to those with lower atomic numbers.
Astrophysics and Atomic Number
In astrophysics, the atomic number is used to study the composition of stars and planets. By analyzing the light from stars (stellar spectroscopy), scientists can determine the atomic numbers of elements present, revealing insights into the processes occurring within stars.
Chemistry FAQ
The atomic number determines the number of protons in an atom’s nucleus, which in turn dictates the number of electrons in a neutral atom. This electron configuration influences the element's chemical properties, such as reactivity, bonding behavior, and oxidation states. For example, elements in the same group have similar valence electron configurations and exhibit similar chemical reactivity despite varying atomic radii. The atomic number is integral in predicting how an element will interact with others, forming compounds and participating in chemical reactions based on its electron arrangement.
Isotopes of an element have the same atomic number but different mass numbers due to variations in the number of neutrons. While the atomic number (number of protons) remains constant, the differing number of neutrons affects the isotope's nuclear stability. Some isotopes are stable, while others are radioactive and decay over time. The stability of an isotope depends on the ratio of protons to neutrons; an imbalance can lead to radioactive decay processes such as alpha, beta, or gamma decay. This variation has implications for applications in dating, medical imaging, and nuclear energy.
The atomic number influences several key trends in the periodic table, including:
-
Atomic Radius: As you move across a period, the atomic radius decreases because the increasing nuclear charge pulls electrons closer to the nucleus. Conversely, moving down a group increases the atomic radius due to the addition of electron shells.
-
Ionization Energy: Generally increases across a period due to greater nuclear charge, making it harder to remove electrons. It decreases down a group as additional electron shells reduce the effective nuclear charge experienced by outer electrons.
-
Electronegativity: Increases across a period as the nucleus attracts electrons more strongly, and decreases down a group due to increased distance from the nucleus and electron shielding.
-
Electron Affinity: Becomes more negative across a period as the added electrons experience a stronger nuclear pull, and less negative down a group due to increased electron shielding.
These trends are a direct consequence of the atomic number’s effect on electron configurations and nuclear charge.
In nuclear reactions, the atomic number is crucial for balancing nuclear equations and ensuring conservation laws are upheld.
-
Fission: When a heavy nucleus undergoes fission, it splits into lighter nuclei with lower atomic numbers, and the atomic number of the products must add up to that of the original nucleus plus any additional particles (e.g., neutrons) emitted during the process.
-
Fusion: In fusion reactions, light nuclei combine to form a heavier nucleus. The total atomic number of the reactants must equal the atomic number of the product nucleus, demonstrating the conservation of atomic number during the fusion process.
Balancing nuclear equations requires accounting for the changes in atomic numbers and ensuring that both the mass number and atomic number are conserved in the reaction.
In synthetic chemistry and material science, understanding atomic number helps predict the formation and properties of new elements or compounds:
-
Synthetic Chemistry: By manipulating the atomic number through nuclear reactions, scientists can create new elements in particle accelerators. The atomic number helps predict the stability, electronic structure, and potential chemical behavior of these new elements.
-
Material Science: The atomic number influences the electronic properties of materials, such as conductivity, magnetism, and optical properties. By selecting elements with specific atomic numbers, scientists can design materials with desired properties for applications in electronics, superconductors, and nanotechnology.
In both fields, knowledge of atomic number aids in tailoring the properties of new materials and elements to meet specific technological and scientific goals.
Conclusion
The atomic number is a fundamental concept that bridges the gap between basic chemical principles and advanced scientific research. From identifying elements and understanding periodic trends to exploring nuclear reactions and synthesizing new elements, the atomic number is integral to many areas of science. Whether you're just starting your studies or delving into advanced topics, a solid understanding of atomic numbers will enhance your comprehension of the physical world and its chemical interactions.