
Proton The Fundamental Building Block of Matter
Protons are one of the essential components of atomic nuclei and play a pivotal role in the structure of matter. This article delves into the nature of protons, their discovery, properties, role in atomic structure, and significance in various scientific fields.
Discovery of the Proton
The proton was discovered by Ernest Rutherford in 1917 during his experiments on the disintegration of nitrogen atoms. By bombarding nitrogen gas with alpha particles, Rutherford observed the emission of hydrogen nuclei, which he identified as a new particle. He named this particle the proton, derived from the Greek word "protos," meaning first, reflecting the idea that hydrogen, with a single proton, is the simplest atom.
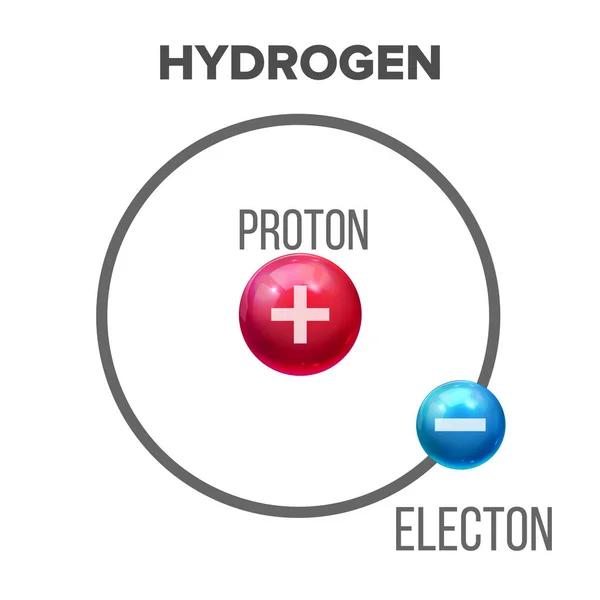
Fundamental Properties of Protons
Charge and Mass
- Charge: Protons carry a positive electrical charge of +1e (elementary charge), which is approximately 1.602 X 10^-19 coulombs.
- Mass: The mass of a proton is about 1.673 X 10^-27 kilograms, which is roughly 1,836 times the mass of an electron.
Location in the Atom
Protons reside in the nucleus of an atom, held together with neutrons by the strong nuclear force, which is one of the four fundamental forces of nature. The number of protons in the nucleus, known as the atomic number, determines the identity of an element.
Composition
Protons are not elementary particles but are composed of three quarks: two up quarks and one down quark, held together by the strong force mediated by gluons. This structure categorizes protons as baryons in the Standard Model of particle physics.
Role of Protons in Atomic Structure
Protons, along with neutrons, form the nucleus of an atom. The arrangement of protons in the nucleus is central to the following aspects of atomic structure:
- Atomic Number: The number of protons defines the atomic number of an element and determines its position in the periodic table. For example, hydrogen has one proton, while carbon has six.
- Chemical Properties: The number of protons influences the chemical properties of an element, as it dictates the number of electrons and their configuration around the nucleus.
- Isotopes: Atoms with the same number of protons but different numbers of neutrons are called isotopes. Isotopes of an element have similar chemical properties but different physical properties, such as stability and atomic mass.
Protons in Chemical Reactions
In chemical reactions, the behavior of atoms is largely governed by the interactions of their electrons. However, the number of protons in the nucleus plays a crucial role in these reactions by determining the element's identity and influencing the electron configuration.
- Acids and Bases: In acid-base chemistry, the proton (H+) is a fundamental species. Acids are proton donors, while bases are proton acceptors. The transfer of protons between molecules is a key mechanism in acid-base reactions.
- Redox Reactions: Protons can also participate in redox reactions, where the transfer of electrons is accompanied by changes in the oxidation state of the elements involved.
Protons in Nuclear Reactions
Protons are integral to nuclear reactions, which involve changes in an atom's nucleus and often result in the transformation of one element into another. Key nuclear reactions involving protons include:
- Nuclear Fusion: In stellar processes, such as those in the sun, protons fuse to form helium nuclei, releasing enormous amounts of energy. Fusion reactions are also the basis for experimental attempts to develop fusion power as a sustainable energy source.
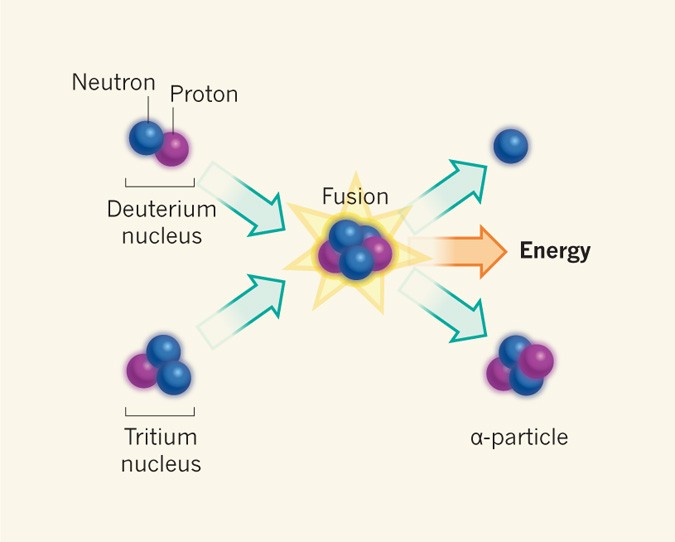
- Nuclear Fission: Although protons do not play a direct role in fission, the balance of protons and neutrons in a nucleus affects its stability and likelihood of undergoing fission.

- Proton Therapy: In medicine, proton beams are used in cancer treatment. Proton therapy targets tumors with high precision, minimizing damage to surrounding healthy tissues.
Significance of Protons in Science and Technology
Protons are fundamental to various scientific and technological advancements:
- Particle Accelerators: Protons are accelerated to high speeds in particle accelerators for experimental research in high-energy physics, leading to discoveries about fundamental particles and forces.
- Hydrogen Economy: Protons, in the form of hydrogen ions, are central to the development of fuel cells and other technologies aimed at creating a sustainable hydrogen economy.
- Cosmic Rays: Protons constitute the majority of cosmic rays, high-energy particles that originate from outer space and provide insights into astrophysical processes and the composition of the universe.
Chemistry FAQ
The number of protons in the nucleus of an atom, known as the atomic number, uniquely identifies an element. Each element on the periodic table has a distinct atomic number, which determines its chemical properties. For instance, hydrogen has one proton, helium has two, and carbon has six. The proton count dictates the element's place in the periodic table and its interactions with other elements.
A proton is composed of three quarks: two up quarks and one down quark. These quarks are held together by the exchange of gluons, which are the carriers of the strong force. The specific combination of quarks gives the proton its charge of +1 (up quarks have a charge of +2/3 each, and down quarks have a charge of -1/3). The quark-gluon interactions within the proton are complex and result in its overall mass and stability, despite the quarks themselves accounting for only a small portion of the proton’s mass; the majority comes from the energy of the gluon field, according to quantum chromodynamics (QCD).
High-energy proton-proton collisions, like those conducted at the Large Hadron Collider (LHC), allow physicists to probe the fundamental forces and particles at very small scales. These collisions can produce a variety of particles and provide insights into the Standard Model of particle physics. For example, the discovery of the Higgs boson in 2012 was a direct result of such collisions. These experiments also help test theories beyond the Standard Model, such as supersymmetry or extra dimensions, by searching for new particles and interactions.
Conclusion
Protons are indispensable to the structure and behavior of matter, influencing everything from the identity of elements to the mechanisms of chemical and nuclear reactions. Their discovery marked a significant milestone in the understanding of atomic structure, and ongoing research continues to reveal the profound implications of protons in various scientific fields. As we deepen our understanding of protons, we unlock new possibilities for technological innovation and a greater comprehension of the universe's fundamental nature.
Here are some useful references you can check out to deepen your understanding of protons:
-
K. S. Krane, Introductory Nuclear Physics (1987)
This textbook provides a detailed introduction to nuclear physics, including protons and their role in the structure of the atomic nucleus. It's a great resource for those seeking a more academic understanding of subatomic particles. -
R.F. E. Rutherford, "Collision of α Particles with Light Atoms," Philosophical Magazine (1919)
Ernest Rutherford's original research paper on the discovery of the proton. It’s a classic historical reference for those interested in the origin of proton discovery. -
P. Atkins and J. de Paula, Atkins' Physical Chemistry (2010)
This textbook offers a comprehensive look at physical chemistry concepts, including atomic structure, quantum mechanics, and the role of protons in chemical reactions. -
A. Zee, Quantum Field Theory in a Nutshell (2010)
For a deeper dive into the quantum aspects of protons, including their quark structure and interactions via gluons, this book is a helpful reference, aimed at advanced readers. -
European Organization for Nuclear Research (CERN)
Website: https://home.cern/
CERN's resources and research papers provide insight into particle physics, including the behavior of protons in high-energy physics experiments.
These references will offer both historical and modern perspectives on protons, from their discovery to their role in cutting-edge physics research.





