
The Power of Beta Particles A Comprehensive Exploration
In the enigmatic realm of particle physics, there exists a fascinating category known as beta particles. These particles, designated as β, are among the fundamental constituents of matter and play pivotal roles in various natural processes and scientific endeavors. In this article, we embark on a journey to uncover the mysteries and intricacies surrounding beta particles, exploring their properties, origins, interactions, and applications.
Understanding Beta Particles
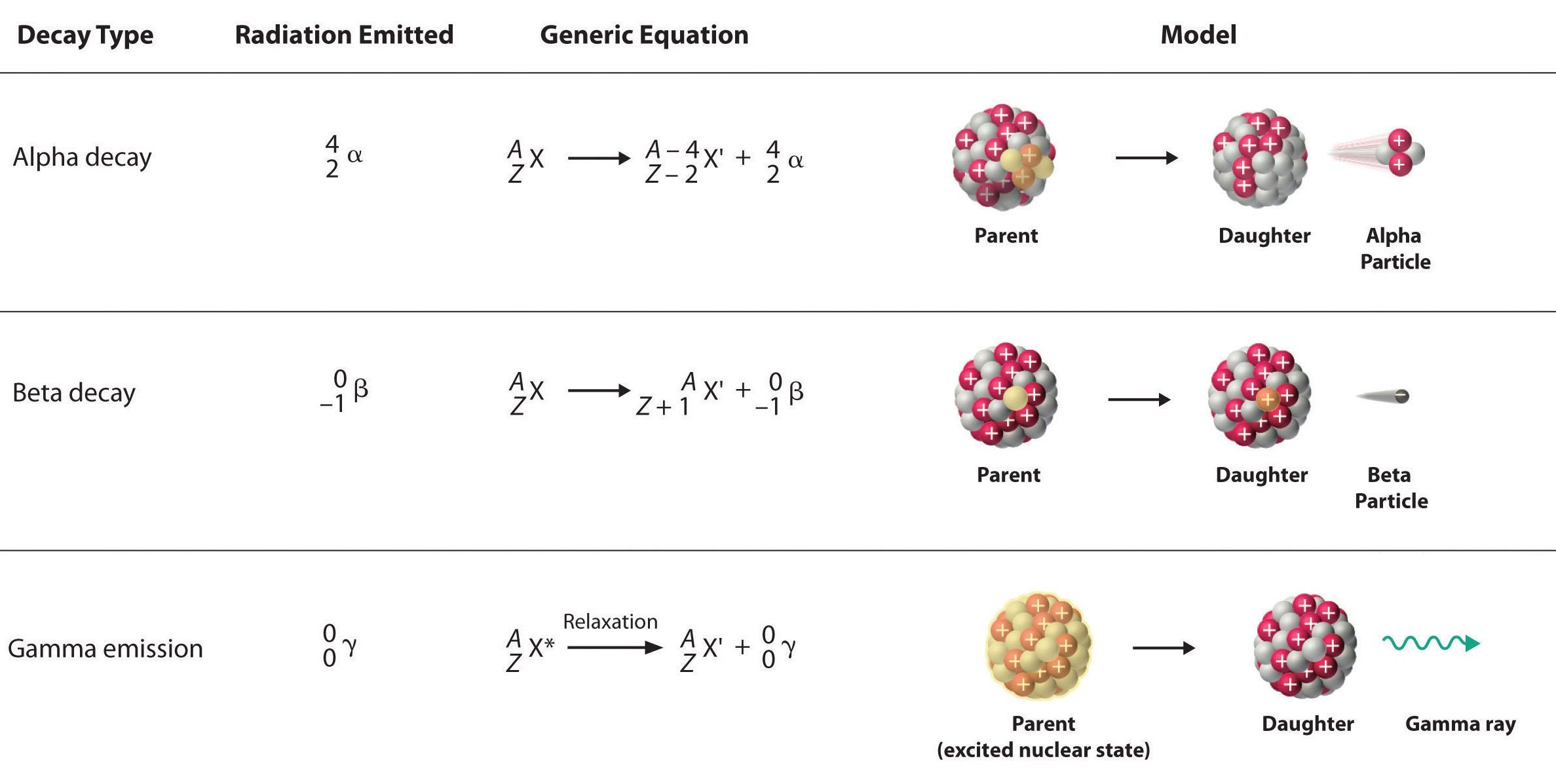
Beta particles are a type of ionizing radiation, meaning they possess sufficient energy to liberate electrons from atoms, thus ionizing them. These particles are commonly encountered in the form of beta decay, a fundamental nuclear process wherein an unstable atomic nucleus undergoes transformation, emitting a beta particle in the process. Beta decay typically occurs in nuclei with an excess of neutrons or protons, seeking stability through the conversion of one into the other.
There are two primary types of beta decay:
-
Beta-minus (β⁻) decay: In this process, a neutron within the nucleus transforms into a proton, emitting an electron (β⁻) and an antineutrino (ν̅ₑ). The atomic number increases by one while the mass number remains unchanged.
-
Beta-plus (β⁺) decay: Also known as positron emission, this process involves the conversion of a proton into a neutron, resulting in the emission of a positron (β⁺) and a neutrino (νₑ). In this case, the atomic number decreases by one while the mass number remains constant.
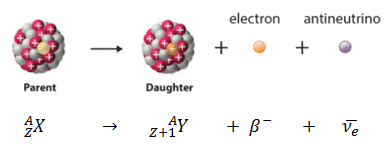
Properties of Beta Particles
Beta particles exhibit distinct characteristics that distinguish them from other forms of radiation:
-
Charge: Beta particles carry an electric charge, with beta-minus particles possessing a negative charge equivalent to -1 elementary charge (e), while beta-plus particles bear a positive charge of +1 e.
-
Mass: Beta particles are much less massive than alpha particles, with beta-minus particles having a mass similar to that of electrons, and beta-plus particles being identical to electrons in mass.
-
Penetrating Power: Compared to alpha particles, beta particles have higher penetrating power but are less penetrating than gamma rays. Beta-minus particles can penetrate a few millimeters of material, while beta-plus particles have a higher energy and can penetrate slightly farther.
Interactions of Beta Particles
As ionizing radiation, beta particles interact with matter through various mechanisms, leading to diverse outcomes:
-
Ionization: Beta particles ionize atoms by colliding with electrons, causing them to be ejected from their orbits. This process contributes to the creation of charged particles and free radicals within the material.
-
Excitation: Beta particles may excite atoms by imparting energy to their electrons, causing them to transition to higher energy states. Subsequent relaxation of these excited atoms can emit characteristic radiation such as X-rays.
-
Scattering: Beta particles can undergo scattering when they interact with the nuclei of atoms in the material. This scattering process alters the direction and energy of the beta particles, influencing their behavior within the medium.
Applications of Beta Particles
Beta particles find diverse applications across various fields, owing to their unique properties and interactions:
-
Medical Imaging and Therapy: Beta-emitting radioisotopes are employed in nuclear medicine for diagnostic imaging and cancer therapy. Radiopharmaceuticals containing beta-emitting isotopes such as iodine-131 and yttrium-90 are utilized in targeted radiation therapies to treat various malignancies.
-
Industrial Radiography: Beta sources are utilized in industrial radiography for non-destructive testing of materials, such as weld inspections and thickness measurements. The penetrating power of beta particles allows for the examination of thicker materials compared to X-rays.
-
Smoke Detection: Beta radiation sources are incorporated into smoke detectors as ionization chambers. When smoke particles enter the chamber, they disrupt the flow of ions produced by the beta source, triggering an alarm.
-
Environmental Monitoring: Beta-emitting isotopes are utilized in environmental monitoring to assess soil and water contamination, track the movement of pollutants, and study ecological processes.
Beta particles FAQ
Beta particles can pose both external and internal radiation hazards. Externally, they can cause skin burns and damage to tissues and eyes. Internally, they can be more harmful if ingested or inhaled, as they can ionize biological molecules, potentially leading to cell damage or cancer.
Protective measures include:
- Shielding: Using materials like acrylic, plastic, or aluminum to absorb beta particles and reduce exposure.
- Distance: Maintaining a safe distance from beta-emitting sources, as the intensity of radiation decreases with distance.
- Containment: Using proper containment and ventilation to prevent inhalation or ingestion of beta-emitting materials.
- Personal Protective Equipment (PPE): Wearing lab coats, gloves, and safety goggles to protect skin and eyes from beta radiation.
Beta particles have various applications in medicine and industry due to their ionizing properties and moderate penetration ability:
-
Medical applications:
- Radiotherapy: Beta-emitting isotopes (e.g., Yttrium-90) are used in targeted radiotherapy to treat cancer by damaging cancerous cells while minimizing exposure to surrounding healthy tissue.
-Diagnostic imaging: Positron Emission Tomography (PET) scans use positron-emitting isotopes (e.g., Fluorine-18) to produce detailed images of metabolic processes in the body.
-
Industrial applications:
- Thickness gauging: Beta particles are used to measure the thickness of materials, such as paper, plastic, or metal, by detecting the amount of beta radiation that passes through the material.
- Radioisotope tracers: Beta-emitting isotopes are used as tracers in various industrial processes to track the flow of substances or detect leaks in pipelines.
Beta decay is a fundamental nuclear process in which a parent nucleus transforms into a daughter nucleus, emitting beta particles in the process. There are three primary modes of beta decay: beta-minus (β⁻), beta-plus (β⁺), and electron capture (EC). In beta-minus decay, a neutron within the nucleus is transformed into a proton, emitting an electron (beta particle) and an antineutrino. In beta-plus decay, a proton decays into a neutron, emitting a positron (positively charged beta particle) and a neutrino. Electron capture involves the capture of an orbital electron by the nucleus, leading to the emission of a neutrino and the conversion of a proton into a neutron.
Beta particles, with their ability to penetrate tissues and deposit energy along their path, find numerous applications in medical imaging and radiation therapy. In positron emission tomography (PET), positron-emitting isotopes such as fluorine-18 decay via beta-plus emission, producing pairs of gamma photons that can be detected and used to reconstruct three-dimensional images of internal organs and biological processes. In radiation therapy, beta-emitting isotopes are employed to selectively target and destroy cancerous cells while minimizing damage to surrounding healthy tissue, offering a non-invasive and targeted approach to cancer treatment.
Beta-delayed neutron emission occurs when a beta-decaying nucleus undergoes additional neutron emission, delaying the release of neutrons relative to the initial beta decay event. This phenomenon is of particular importance in nuclear astrophysics, where it affects the synthesis of heavy elements in stellar environments such as supernovae and neutron star mergers. Beta-delayed neutron emission can influence the neutron-to-proton ratio in nuclear reactions, affecting the production of elements beyond iron in the periodic table. By studying the properties of beta-delayed neutron emitters and their decay pathways, scientists gain insights into the nucleosynthesis processes responsible for the abundance of elements in the universe and the origins of heavy elements.
Conclusion
Beta particles represent a captivating aspect of particle physics, embodying the intricate dance of fundamental particles within the fabric of the universe. From their origins in nuclear decay to their manifold applications in science and technology, beta particles continue to intrigue and inspire researchers, offering profound insights into the nature of matter and energy. As our understanding of these enigmatic particles deepens, so too does our ability to harness their power for the betterment of humanity.
Here are some useful references for further exploration on beta particles:
-
Nuclear Physics: Principles and Applications by John Lilley
This textbook provides a detailed explanation of nuclear physics concepts, including beta decay and the behavior of beta particles in various applications. -
"Beta Decay" – HyperPhysics, Georgia State University
HyperPhysics offers clear, concise explanations of beta decay processes and the nature of beta particles. -
The Health Physics Society
Health Physics Society provides information on radiation safety, including detailed discussions on beta particles, their health risks, and protective measures. -
International Atomic Energy Agency (IAEA) – Radiation and Nuclear Safety
IAEA has various resources related to radiation types, including beta particles, and their applications in medical and industrial fields. -
"Introduction to Radiological Physics and Radiation Dosimetry" by Frank Herbert Attix
This book provides an in-depth look at radiation physics, including the ionizing effects of beta particles and how they are measured. -
U.S. Environmental Protection Agency (EPA) – Radiation: Beta Particles
EPA Radiation offers accessible information on the properties of beta particles, their sources, and safety guidelines for handling them.
These resources should give you comprehensive insights into the science and applications of beta particles, as well as the precautions necessary for working with radiation.





