
Volume Measurements in Chemical Analysis and Use of Glassware
Accurate volume measurement is a fundamental aspect of chemical analysis, ensuring the precision and reliability of experimental results. The proper use of glassware is critical in achieving this accuracy. This article delves into the importance of volume measurements, the types of glassware used, and best practices for using and maintaining these tools.
Importance of Accurate Volume Measurements
Volume measurement is crucial in chemical analysis for several reasons:
- Concentration Determination: Accurate volumes are essential for preparing solutions of precise concentrations.
- Reaction Stoichiometry: Ensuring the correct proportions of reactants in a chemical reaction.
- Standardization: Consistency in experiments, allowing for reproducibility and comparability of results.
- Titration: Precise volume measurements are critical for determining the concentration of unknown solutions.
Types of Glassware for Volume Measurement
Several types of glassware are commonly used in laboratories for measuring volumes. Each type has specific applications, advantages, and limitations.
1. Beakers
Description: Cylindrical glass containers with a flat bottom, available in various sizes, typically with a spout for pouring.
Uses:
- Mixing and stirring solutions.
- Rough volume measurements.
Advantages:
- Easy to use.
- Versatile.
Limitations:
- Not suitable for precise volume measurements due to a lack of calibration marks.

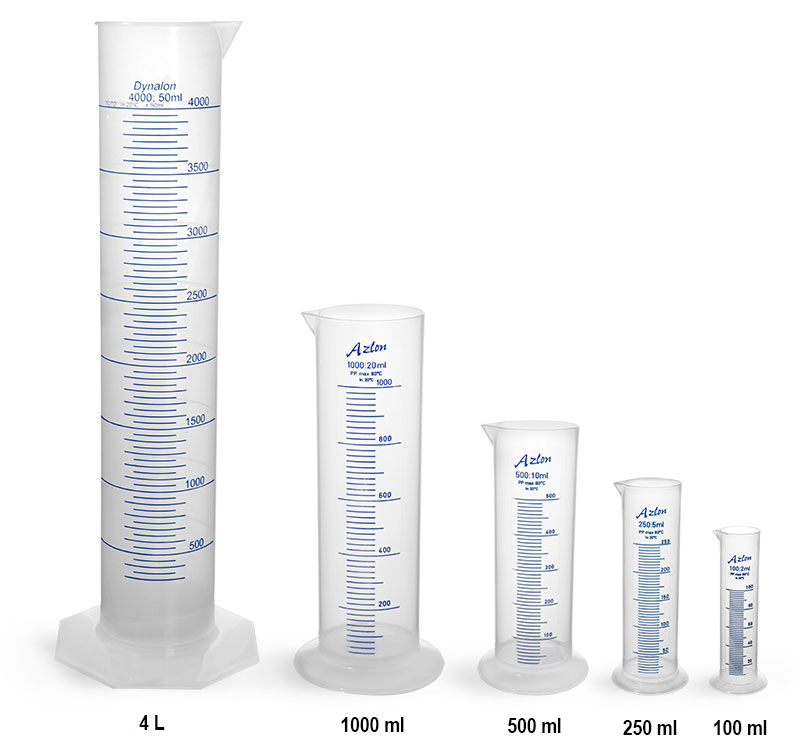
2. Graduated Cylinders
Description: Tall, cylindrical glassware with volume markings along the side.
Uses:
- Measuring out specific volumes of liquids.
Advantages:
- More accurate than beakers.
- Available in various sizes to suit different volume requirements.
Limitations:
- Still less accurate compared to volumetric flasks or pipettes.
3. Volumetric Flasks
Description: Flasks with a precise calibration mark on the neck.
Uses:
- Preparing solutions of exact volumes and concentrations.
Advantages:
- High accuracy and precision.
- Used for making standard solutions.
Limitations:
- Limited to specific volumes (e.g., 50 mL, 100 mL, 250 mL).

4. Pipettes
Description: Tubes used to transport a measured volume of liquid, available in several types including volumetric, graduated, and micropipettes.
Uses:
- Transferring precise volumes of liquids.
Advantages:
- High precision, especially with volumetric and micropipettes.
- Essential for titration and sample preparation.
Limitations:
- Requires careful handling and calibration.

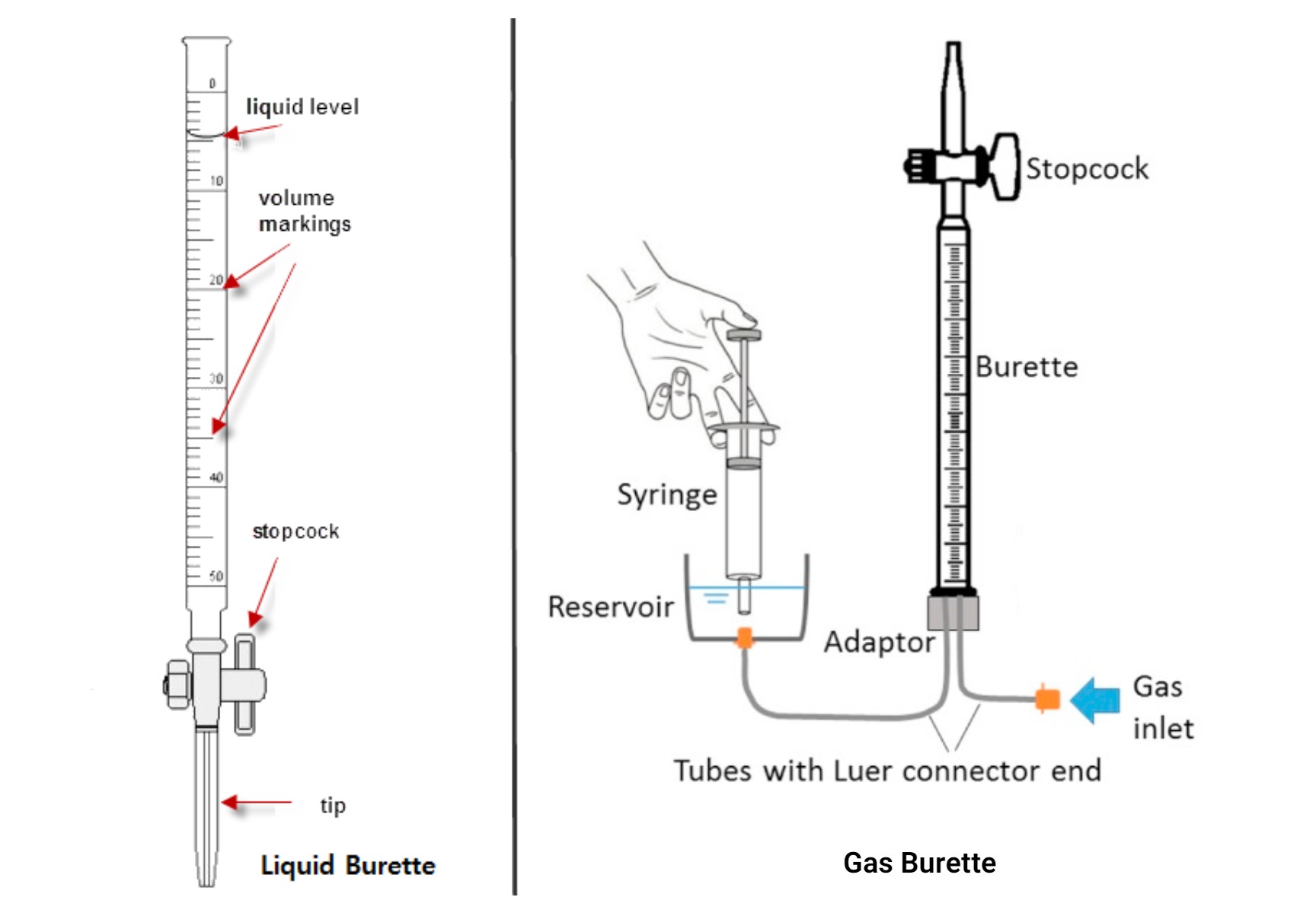
5. Burettes
Description: Long, graduated tubes with a stopcock at the bottom.
Uses:
- Delivering known volumes of liquid, especially in titrations.
Advantages:
- High precision and control over liquid delivery.
Limitations:
- Requires proper setup and handling to ensure accuracy.
Best Practices for Using Glassware
To achieve the best results in volume measurement, it is important to follow these best practices:
1. Calibration and Maintenance
- Regular Calibration: Ensure all glassware is calibrated periodically to maintain accuracy.
- Cleaning: Clean glassware thoroughly before and after use to prevent contamination and inaccurate readings.
- Inspection: Check for chips, cracks, and other damages that might affect measurements.
2. Proper Technique
- Reading Meniscus: When measuring liquids, read the bottom of the meniscus at eye level to avoid parallax errors.
- Pipetting: Use a pipette bulb or pump to draw liquid into pipettes; avoid mouth pipetting for safety and accuracy.
- Burette Use: Ensure the burette is vertical and free of air bubbles; deliver liquid slowly and steadily.
3. Handling and Storage
- Handling: Handle glassware with care to avoid breakage and ensure longevity.
- Storage: Store glassware in a clean, dry place, preferably in a way that prevents dust accumulation and physical damage.
Common Errors and How to Avoid Them
1. Parallax Error
Description: Misreading the meniscus level due to viewing at an angle.
Prevention: Always read the meniscus at eye level.

2. Contamination
Description: Residual chemicals affecting measurements.
Prevention: Clean glassware thoroughly and rinse with the solution being measured.
3. Temperature Variations
Description: Liquid volumes can expand or contract with temperature changes.
Prevention: Perform measurements at a consistent temperature, ideally at standard laboratory conditions (20°C).
Chemistry FAQ
Class A and Class B are classifications that indicate the tolerance levels of volumetric glassware:
- Class A Glassware:
- Accuracy: Higher accuracy with tighter tolerance limits.
- Use Cases: Critical measurements where precision is paramount, such as standard solution preparation and analytical procedures.
- Identification: Usually marked with an "A" or "AS" (for analytical standard) and often has a batch or individual calibration certificate.
- Class B Glassware:
- Accuracy: Lower accuracy with wider tolerance limits.
- Use Cases: Routine measurements where extreme precision is not as critical, such as general laboratory work and educational purposes.
- Identification: Typically marked with a "B" and does not usually come with a calibration certificate.
Meniscus reading errors occur when the liquid level is not read correctly at eye level, leading to either overestimation or underestimation of the actual volume. This can significantly impact the accuracy of measurements, especially in small volumes. To minimize these errors:
- Correct Technique: Always read the bottom of the meniscus at eye level for transparent liquids and the top of the meniscus for opaque or colored liquids.
- Consistent Viewing Angle: Ensure that the glassware is on a level surface and viewed straight on, not at an angle.
- Calibration: Use calibrated glassware with clear, etched markings to improve reading precision.
- Training: Proper training of personnel in the correct reading of the meniscus.
The choice of pipette depends on the volume to be measured and the required precision:
- Volumetric Pipettes:
- Precision: High precision for single, fixed volumes.
- Use Cases: Preparation of standard solutions and titrations where exact volume delivery is critical.
- Graduated Pipettes:
- Flexibility: Can measure and deliver variable volumes, but with less precision compared to volumetric pipettes.
- Use Cases: General laboratory tasks where flexibility in volume measurement is needed and high precision is less critical.
- Micropipettes:
- Precision and Range: Extremely high precision for small volumes, typically in the microliter range.
- Use Cases: Molecular biology, biochemistry, and analytical chemistry where precise measurement of small volumes is essential.
When choosing a pipette, consider the required accuracy, the volume range, the type of liquid being handled, and the specific requirements of the analytical procedure.
Conclusion
Accurate volume measurement is vital for reliable chemical analysis. Understanding the types of glassware and their appropriate uses, along with adhering to best practices, ensures precision and consistency in laboratory work. Proper maintenance, handling, and calibration of glassware are integral to achieving the highest standards of accuracy in volume measurements. By following these guidelines, scientists and technicians can maintain the integrity of their experimental results and contribute to the advancement of scientific knowledge.





